Determination of the relative economic impact of different molecular-based laboratory algorithms for respiratory viral pathogen detection, including Pandemic (H1N1), using a secure web based platform
- PMID: 21645365
- PMCID: PMC3123288
- DOI: 10.1186/1743-422X-8-277
Determination of the relative economic impact of different molecular-based laboratory algorithms for respiratory viral pathogen detection, including Pandemic (H1N1), using a secure web based platform
Abstract
Background: During period of crisis, laboratory planners may be faced with a need to make operational and clinical decisions in the face of limited information. To avoid this dilemma, our laboratory utilizes a secure web based platform, Data Integration for Alberta Laboratories (DIAL) to make near real-time decisions.This manuscript utilizes the data collected by DIAL as well as laboratory test cost modeling to identify the relative economic impact of four proposed scenarios of testing for Pandemic H1N1 (2009) and other respiratory viral pathogens.
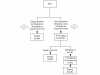
Methods: Historical data was collected from the two waves of the pandemic using DIAL. Four proposed molecular testing scenarios were generated: A) Luminex respiratory virus panel (RVP) first with/without US centers for Disease Control Influenza A Matrix gene assay (CDC-M), B) CDC-M first with/without RVP, C) RVP only, and D) CDC-M only. Relative cost estimates of different testing algorithm were generated from a review of historical costs in the lab and were based on 2009 Canadian dollars.
Results: Scenarios A and B had similar costs when the rate of influenza A was low (< 10%) with higher relative cost in Scenario A with increasing incidence. Scenario A provided more information about mixed respiratory virus infection as compared with Scenario B.
Conclusions: No one approach is applicable to all conditions. Testing costs will vary depending on the test volume, prevalence of influenza A strains, as well as other circulating viruses and a more costly algorithm involving a combination of different tests may be chosen to ensure that tests results are returned to the clinician in a quicker manner. Costing should not be the only consideration for determination of laboratory algorithms.
Figures




References
-
- Public health agency of Canada. The Canadian Pandemic Influenza Plan for the Health Sector. 2011. http://www.phac-aspc.gc.ca/cpip-pclcpi/
-
- Pabbaraju K, Wong S, Wong AA, Appleyard GD, Chui L, Pang XL, Yanow SK, Fonseca K, Lee BE, Fox JD. et al.Design and validation of real-time reverse transcription-PCR assays for detection of pandemic (H1N1) 2009 virus. J Clin Microbiol. 2009;47:3454–3460. doi: 10.1128/JCM.01103-09. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

