Sphingosine-1-phosphate promotes the differentiation of human umbilical cord mesenchymal stem cells into cardiomyocytes under the designated culturing conditions
- PMID: 21645412
- PMCID: PMC3127825
- DOI: 10.1186/1423-0127-18-37
Sphingosine-1-phosphate promotes the differentiation of human umbilical cord mesenchymal stem cells into cardiomyocytes under the designated culturing conditions
Abstract
Background: It is of growing interest to develop novel approaches to initiate differentiation of mesenchymal stem cells (MSCs) into cardiomyocytes. The purpose of this investigation was to determine if Sphingosine-1-phosphate (S1P), a native circulating bioactive lipid metabolite, plays a role in differentiation of human umbilical cord mesenchymal stem cells (HUMSCs) into cardiomyocytes. We also developed an engineered cell sheet from these HUMSCs derived cardiomyocytes by using a temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm) cell sheet technology.
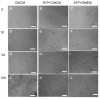
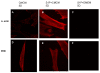
Methods: Cardiomyogenic differentiation of HUMSCs was performed by culturing these cells with either designated cardiomyocytes conditioned medium (CMCM) alone, or with 1 μM S1P; or DMEM with 10% FBS + 1 μM S1P. Cardiomyogenic differentiation was determined by immunocytochemical analysis of expression of cardiomyocyte markers and patch clamping recording of the action potential.
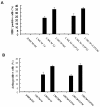
Results: A cardiomyocyte-like morphology and the expression of α-actinin and myosin heavy chain (MHC) proteins can be observed in both CMCM culturing or CMCM+S1P culturing groups after 5 days' culturing, however, only the cells in CMCM+S1P culture condition present cardiomyocyte-like action potential and voltage gated currents. A new approach was used to form PIPAAm based temperature-responsive culture surfaces and this successfully produced cell sheets from HUMSCs derived cardiomyocytes.
Conclusions: This study for the first time demonstrates that S1P potentiates differentiation of HUMSCs towards functional cardiomyocytes under the designated culture conditions. Our engineered cell sheets may provide a potential for clinically applicable myocardial tissues should promote cardiac tissue engineering research.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

