SLAIN2 links microtubule plus end-tracking proteins and controls microtubule growth in interphase
- PMID: 21646404
- PMCID: PMC3115796
- DOI: 10.1083/jcb.201012179
SLAIN2 links microtubule plus end-tracking proteins and controls microtubule growth in interphase
Abstract
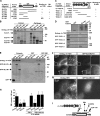
The ends of growing microtubules (MTs) accumulate a set of diverse factors known as MT plus end-tracking proteins (+TIPs), which control microtubule dynamics and organization. In this paper, we identify SLAIN2 as a key component of +TIP interaction networks. We showed that the C-terminal part of SLAIN2 bound to end-binding proteins (EBs), cytoplasmic linker proteins (CLIPs), and CLIP-associated proteins and characterized in detail the interaction of SLAIN2 with EB1 and CLIP-170. Furthermore, we found that the N-terminal part of SLAIN2 interacted with ch-TOG, the mammalian homologue of the MT polymerase XMAP215. Through its multiple interactions, SLAIN2 enhanced ch-TOG accumulation at MT plus ends and, as a consequence, strongly stimulated processive MT polymerization in interphase cells. Depletion or disruption of the SLAIN2-ch-TOG complex led to disorganization of the radial MT array. During mitosis, SLAIN2 became highly phosphorylated, and its interaction with EBs and ch-TOG was inhibited. Our study provides new insights into the molecular mechanisms underlying cell cycle-specific regulation of MT polymerization and the organization of the MT network.
Figures





References
-
- Akhmanova A., Hoogenraad C.C., Drabek K., Stepanova T., Dortland B., Verkerk T., Vermeulen W., Burgering B.M., De Zeeuw C.I., Grosveld F., Galjart N. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 104:923–935 10.1016/S0092-8674(01)00288-4 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

