Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke
- PMID: 21646410
- PMCID: PMC3180573
- DOI: 10.1113/jphysiol.2011.210294
Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke
Abstract
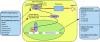
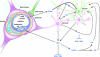
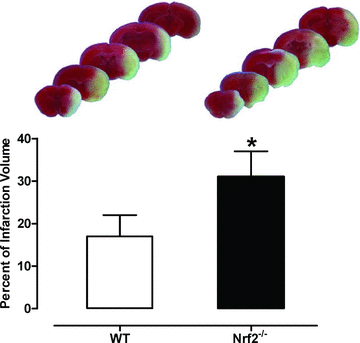
Endogenous defence mechanisms by which the brain protects itself against noxious stimuli and recovers from ischaemic damage are a key target of stroke research. The loss of viable brain tissue in the ischaemic core region after stroke is associated with damage to the surrounding area known as the penumbra. Activation of the redox-sensitive transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) plays a pivotal role in the cellular defence against oxidative stress via transcriptional upregulation of phase II defence enzymes and antioxidant stress proteins. Although recent evidence implicates Nrf2 in neuroprotection, it is not known whether activation of this pathway within the neurovascular unit protects the brain against blood-brain barrier breakdown and cerebrovascular inflammation. Targeting the neurovascular unit should provide novel insights for effective treatment strategies and facilitate translation of experimental findings into clinical therapy. This review focuses on the cytoprotective role of Nrf2 in stroke and examines the evidence that the Nrf2-Keap1 defence pathway may serve as a therapeutic target for neurovascular protection.
Figures
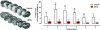
References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12:125–169. - PubMed
-
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

