Regulatory circuitry governing fungal development, drug resistance, and disease
- PMID: 21646428
- PMCID: PMC3122626
- DOI: 10.1128/MMBR.00045-10
Regulatory circuitry governing fungal development, drug resistance, and disease
Abstract
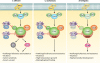
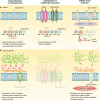
Pathogenic fungi have become a leading cause of human mortality due to the increasing frequency of fungal infections in immunocompromised populations and the limited armamentarium of clinically useful antifungal drugs. Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus are the leading causes of opportunistic fungal infections. In these diverse pathogenic fungi, complex signal transduction cascades are critical for sensing environmental changes and mediating appropriate cellular responses. For C. albicans, several environmental cues regulate a morphogenetic switch from yeast to filamentous growth, a reversible transition important for virulence. Many of the signaling cascades regulating morphogenesis are also required for cells to adapt and survive the cellular stresses imposed by antifungal drugs. Many of these signaling networks are conserved in C. neoformans and A. fumigatus, which undergo distinct morphogenetic programs during specific phases of their life cycles. Furthermore, the key mechanisms of fungal drug resistance, including alterations of the drug target, overexpression of drug efflux transporters, and alteration of cellular stress responses, are conserved between these species. This review focuses on the circuitry regulating fungal morphogenesis and drug resistance and the impact of these pathways on virulence. Although the three human-pathogenic fungi highlighted in this review are those most frequently encountered in the clinic, they represent a minute fraction of fungal diversity. Exploration of the conservation and divergence of core signal transduction pathways across C. albicans, C. neoformans, and A. fumigatus provides a foundation for the study of a broader diversity of pathogenic fungi and a platform for the development of new therapeutic strategies for fungal disease.
Figures
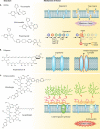


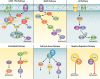
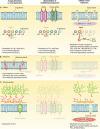


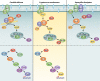





References
-
- Al-Dhaheri R. S., Douglas L. J. 2010. Apoptosis in Candida biofilms exposed to amphotericin B. J. Med. Microbiol. 59:149–157 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

