cis-antisense RNA, another level of gene regulation in bacteria
- PMID: 21646430
- PMCID: PMC3122628
- DOI: 10.1128/MMBR.00032-10
cis-antisense RNA, another level of gene regulation in bacteria
Abstract
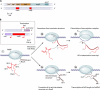
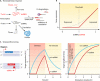
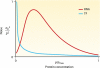
A substantial amount of antisense transcription is a hallmark of gene expression in eukaryotes. However, antisense transcription was first demonstrated in bacteria almost 50 years ago. The transcriptomes of bacteria as different as Helicobacter pylori, Bacillus subtilis, Escherichia coli, Synechocystis sp. strain PCC6803, Mycoplasma pneumoniae, Sinorhizobium meliloti, Geobacter sulfurreducens, Vibrio cholerae, Chlamydia trachomatis, Pseudomonas syringae, and Staphylococcus aureus have now been reported to contain antisense RNA (asRNA) transcripts for a high percentage of genes. Bacterial asRNAs share functional similarities with trans-acting regulatory RNAs, but in addition, they use their own distinct mechanisms. Among their confirmed functional roles are transcription termination, codegradation, control of translation, transcriptional interference, and enhanced stability of their respective target transcripts. Here, we review recent publications indicating that asRNAs occur as frequently in simple unicellular bacteria as they do in higher organisms, and we provide a comprehensive overview of the experimentally confirmed characteristics of asRNA actions and intimately linked quantitative aspects. Emerging functional data suggest that asRNAs in bacteria mediate a plethora of effects and are involved in far more processes than were previously anticipated. Thus, the functional impact of asRNAs should be considered when developing new strategies against pathogenic bacteria and when optimizing bacterial strains for biotechnology.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

