Microbial ecology of the dark ocean above, at, and below the seafloor
- PMID: 21646433
- PMCID: PMC3122624
- DOI: 10.1128/MMBR.00039-10
Microbial ecology of the dark ocean above, at, and below the seafloor
Abstract
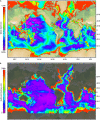
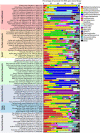
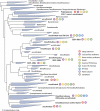
The majority of life on Earth--notably, microbial life--occurs in places that do not receive sunlight, with the habitats of the oceans being the largest of these reservoirs. Sunlight penetrates only a few tens to hundreds of meters into the ocean, resulting in large-scale microbial ecosystems that function in the dark. Our knowledge of microbial processes in the dark ocean-the aphotic pelagic ocean, sediments, oceanic crust, hydrothermal vents, etc.-has increased substantially in recent decades. Studies that try to decipher the activity of microorganisms in the dark ocean, where we cannot easily observe them, are yielding paradigm-shifting discoveries that are fundamentally changing our understanding of the role of the dark ocean in the global Earth system and its biogeochemical cycles. New generations of researchers and experimental tools have emerged, in the last decade in particular, owing to dedicated research programs to explore the dark ocean biosphere. This review focuses on our current understanding of microbiology in the dark ocean, outlining salient features of various habitats and discussing known and still unexplored types of microbial metabolism and their consequences in global biogeochemical cycling. We also focus on patterns of microbial diversity in the dark ocean and on processes and communities that are characteristic of the different habitats.
Figures













References
-
- Abe F., Horikoshi K. 2001. The biotechnological potential of piezophiles. Trends Biotechnol. 19:102–108 - PubMed
-
- Agogué H., Brink M., Dinasquet J., Herndl G. J. 2008. Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic. Nature 456:788–791 - PubMed
-
- Aiello I. W., Bekins B. 2008. Milankovitch-scale correlations between deeply buried microbial populations and biogenic ooze lithology. Eos Trans. AGU 89(Fall Meet. Suppl.):B51F–08
-
- Alain K., et al. 2002. Marinitoga piezophila sp. nov., a rod-shaped, thermo-piezophilic bacterium isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 52:1331–1339 - PubMed
-
- Alain K., et al. 2002. Caminicella sporogenes gen. nov., sp. nov., a novel thermophilic spore-forming bacterium isolated from an East-Pacific Rise hydrothermal vent. Int. J. Syst. Evol. Microbiol. 52:1621–1628 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

