Direct conversion of human fibroblasts to dopaminergic neurons
- PMID: 21646515
- PMCID: PMC3121829
- DOI: 10.1073/pnas.1105135108
Direct conversion of human fibroblasts to dopaminergic neurons
Abstract
Recent reports demonstrate that somatic mouse cells can be directly converted to other mature cell types by using combined expression of defined factors. Here we show that the same strategy can be applied to human embryonic and postnatal fibroblasts. By overexpression of the transcription factors Ascl1, Brn2, and Myt1l, human fibroblasts were efficiently converted to functional neurons. We also demonstrate that the converted neurons can be directed toward distinct functional neurotransmitter phenotypes when the appropriate transcriptional cues are provided together with the three conversion factors. By combining expression of the three conversion factors with expression of two genes involved in dopamine neuron generation, Lmx1a and FoxA2, we could direct the phenotype of the converted cells toward dopaminergic neurons. Such subtype-specific induced neurons derived from human somatic cells could be valuable for disease modeling and cell replacement therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




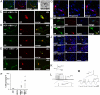
Comment in
- Regen Med. 2011 Nov;6(6):685-7
References
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

