2D NMR-spectroscopic screening reveals polyketides in ladybugs
- PMID: 21646540
- PMCID: PMC3116430
- DOI: 10.1073/pnas.1107020108
2D NMR-spectroscopic screening reveals polyketides in ladybugs
Abstract
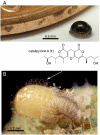
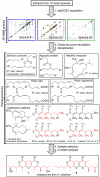
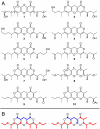
Small molecules of biological origin continue to yield the most promising leads for drug design, but systematic approaches for exploring nature's cache of structural diversity are lacking. Here, we demonstrate the use of 2D NMR spectroscopy to screen a library of biorationally selected insect metabolite samples for partial structures indicating the presence of new chemical entities. This NMR-spectroscopic survey enabled detection of novel compounds in complex metabolite mixtures without prior fractionation or isolation. Our screen led to discovery and subsequent isolation of two families of tricyclic pyrones in Delphastus catalinae, a tiny ladybird beetle that is employed commercially as a biological pest control agent. The D. catalinae pyrones are based on 23-carbon polyketide chains forming 1,11-dioxo-2,6,10-trioxaanthracene and 4,8-dioxo-1,9,13-trioxaanthracene derivatives, representing ring systems not previously found in nature. This study highlights the utility of 2D NMR-spectroscopic screening for exploring nature's structure space and suggests that insect metabolomes remain vastly underexplored.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ortholand JY, Ganesan A. Natural products and combinatorial chemistry: Back to the future. Curr Opin Chem Biol. 2004;8:271–280. - PubMed
-
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. - PubMed
-
- Rapaka RS, et al. Drug Addiction. New York: Springer; 2008. Drug discovery from natural sources; pp. 17–39.
-
- Gronquist M, Schroeder FC, Lew M, Hung-Wen L. Comprehensive Natural Products II. Oxford, UK: Elsevier; 2010. Insect natural products; pp. 67–108.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

