Asymmetric synthesis of tertiary thiols and thioethers
- PMID: 21647256
- PMCID: PMC3107483
- DOI: 10.3762/bjoc.7.68
Asymmetric synthesis of tertiary thiols and thioethers
Abstract
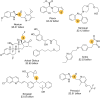
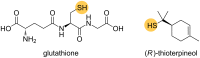
Enantiomerically pure tertiary thiols provide a major synthetic challenge, and despite the importance of chiral sulfur-containing compounds in biological and medicinal chemistry, surprisingly few effective methods are suitable for the asymmetric synthesis of tertiary thiols. This review details the most practical of the methods available.
Keywords: asymmetric synthesis; organolithium; substitution; sulfur; thiol.
Figures
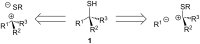
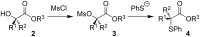
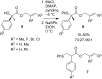
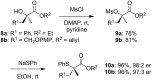
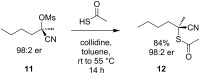
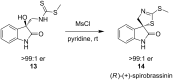
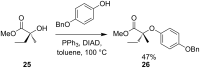
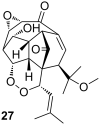
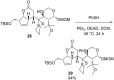
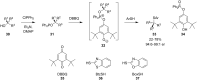
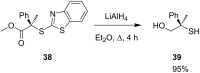
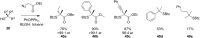
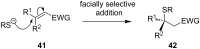
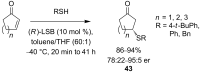
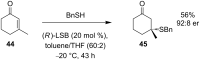
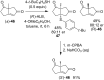


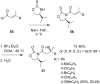
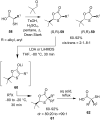
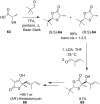
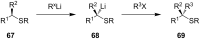
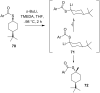
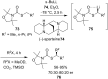

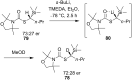
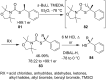

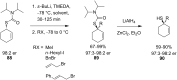
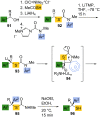
References
-
- 2009 Top 200 Branded Drugs by Retail Dollars. Drug Topics; 2010. Available from: http://drugtopics.modernmedicine.com/drugtopics/data/articlestandard//dr....
-
- Cremlyn R J. An Introduction to Organosulfur Chemistry. Chichester, U.K.: John Wiley & Sons Ltd.; 1996.
-
- Toru T, Bolm C, editors. Organosulfur Chemistry in Asymmetric Synthesis. Weinheim, Germany: Wiley-VCH; 2008.
-
- Pellissier H. Tetrahedron. 2006;62:5559–5601. doi: 10.1016/j.tet.2006.03.093. - DOI
LinkOut - more resources
Full Text Sources
