The crucial role of collagen-binding integrins in maintaining the mechanical properties of human scleral fibroblasts-seeded collagen matrix
- PMID: 21647271
- PMCID: PMC3107998
The crucial role of collagen-binding integrins in maintaining the mechanical properties of human scleral fibroblasts-seeded collagen matrix
Abstract
Purpose: The aim of this study was to identify the presence of collagen-binding integrin subunits in human scleral fibroblasts (HSFs) and investigate their actual functions in maintaining the mechanical creep properties of the HSFs-seeded collagen matrix.
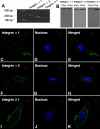
Methods: Primary HSFs were cultured in vitro. Reverse- transcription PCR was used to detect mRNA expression of integrin α1, α2, and β1 subunits in HSFs. In addition, western blot analysis and immunofluorescence were used to detect their protein in HSFs. Monoclonal antibodies were applied directly against the extracellular domains of integrin subunits in HSFs cultured in the three-dimensional collagen gels to block the interaction between HSFs and the extracellular collagen matrix. The effects of anti-integrin antibodies on HSFs morphology in collagen gel were observed. The effects of the added antibodies on fibroblast-mediated collagen gels' contraction were evaluated. Furthermore, the changes in mechanical creep properties of collagen gel were measured by a biomechanics test instrument.
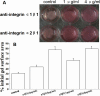
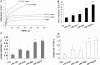
Results: The mRNA and protein expressions of collagen-binding integrin α1, α2, and β1 subunits were present in HSFs. The elongated bipolar cells converted to spherical shapes after 6 h after the addition of integrin α1β1 and α2β1 antibody. The blocking of integrin α1β1 and α2β1 subunits noticeably decreased the contraction in the collagen gels. In addition, all samples were subjected to a constantly applied load of 0.03 N for 600 s. The blocking of integrin α1β1 and α2β1 subunits also induced increases in the values of final extension, creep extension, and creep rate, compared to those of the controls (p<0.01). Furthermore, the creep elements were significantly increased with the augmentation of the integrin antibody dose (p<0.01). The final extension of the integrin α2β1 antibody (1 μg/ml or 4 μg/ml) group was significantly higher compared to that of the integrin α1β1 antibody (1 μg/ml or 4 μg/ml) group (p<0.01). However, the creep extension and creep rate of the integrin α2β1 antibody (1 μg/ml or 4 μg/ml) group were not significantly different from those in the integrin α1β1 antibody (1 μg/ml or 4 μg/ml) group (p>0.05).
Conclusions: Our findings suggested that HSF integrin α1β1 and α2β1 participated in maintaining the mechanical creep properties of the HSFs-seeded collagen matrix. Furthermore, integrin α2β1 might play a more crucial role in maintaining the mechanical creep properties of the collagen matrix than does integrin α1β1.
Figures

References
-
- Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–81. - PubMed
-
- Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82:185–200. - PubMed
-
- Cashwell LF, Martin CA. Axial length decrease accompanying successful glaucoma filtration surgery. Ophthalmology. 1999;106:2307–11. - PubMed
-
- Leydolt C, Findl O, Drexler W. Effects of change in intraocular pressure on axial eye length and lens position. Eye. 2008;22:657–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
