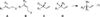Enantioselective construction of all-carbon quaternary centers by branch-selective Pd-catalyzed allyl-allyl cross-coupling
- PMID: 21648464
- PMCID: PMC3131072
- DOI: 10.1021/ja2039248
Enantioselective construction of all-carbon quaternary centers by branch-selective Pd-catalyzed allyl-allyl cross-coupling
Abstract
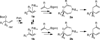
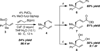
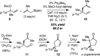
The Pd-catalyzed cross-coupling of racemic tertiary allylic carbonates and allylboronates is described. This reaction generates all-carbon quaternary centers in a highly regioselective and enantioselective fashion. The outcome of these reactions is consistent with a process that proceeds by way of 3,3'-reductive elimination of bis(η(1)-allyl)palladium intermediates. Strategies for distinguishing the product alkenes and application to the synthesis of (+)-α-cuparenone are also described.
Figures
References
-
- Das JP, Marek I. Chem. Commun. 2011;47:4593. - PubMed
- Cozzi PG, Hilgraf R, Zimmermann N. Eur. J. Org. Chem. 2007;5969
- Trost BM, Jiang C. Synthesis. 2006;369
- Christoffers J, Baro A. Adv. Synth. Catal. 2005;447:1473.
- Douglas CJ, Overman LE. Proc. Natl. Acad. Sci. USA. 2004;101:5363. - PMC - PubMed
- Corey EJ, Guzman-Perez A. Angew. Chem. Int. Ed. 1998;37:388. - PubMed
-
- Overman LE. Pure & Appl. Chem. 1994;66:1423.
- Shibasaki M, Boden C, Kojima A. Tetrahedron. 1997;53:7371.
- Shibasaki M, Erasmus MV, Ohshima T. Adv. Synth. Catal. 2004;346:1533.
-
-
Review: Bellina F, Rossi R. Chem. Rev. 2010;110:1082. Selected references: Liao X, Weng Z, Hartwig JF. J. Am. Chem. Soc. 2008;130:195. Chen G, Kwong FY, Chan HO, Yu W, Chan ASC. Chem. Commun. 2006;1413 Hamada T, Chieffi A, Åhman J, Buchwald SL. J. Am. Chem. Soc. 2002;124:1261. Spielvogel DJ, Buchwald SL. J. Am. Chem. Soc. 2002;124:3500. Lee S, Hartwig JF. J. Org. Chem. 2001;66:3402. Åhman J, Wolfe JP, Troutman MV, Palucki M, Buchwald SL. J. Am. Chem. Soc. 1998;120:1918.
-
-
-
Review: Mohr JT, Stoltz BM. Chem. Asian J. 2007;1476 Braun M, Meier T. Angew. Chem. Int. Ed. 2006;45:6952. Selected references: Mohr JT, Behenna DC, Harned AM, Stoltz BM. Angew. Chem. Int. Ed. 2005;44:6924. Trost BM, Schroeder GM. Chem. Eur. J. 2005;11:174. Trost BM, Xu J. J. Am. Chem. Soc. 2005;127:2846. Behenna DC, Stoltz BM. J. Am. Chem. Soc. 2004;126:15044. You S, Hou X, Dai L, Zhu X. Org. Lett. 2001;3:149. Trost BM, Schroeder GM. J. Am. Chem. Soc. 1999;121:6759.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous