Calcitonin and calcitonin receptor-like receptors: common themes with family B GPCRs?
- PMID: 21649645
- PMCID: PMC3415637
- DOI: 10.1111/j.1476-5381.2011.01525.x
Calcitonin and calcitonin receptor-like receptors: common themes with family B GPCRs?
Abstract
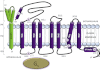
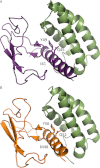
The calcitonin receptor (CTR) and calcitonin receptor-like receptor (CLR) are two of the 15 human family B (or Secretin-like) GPCRs. CTR and CLR are of considerable biological interest as their pharmacology is moulded by interactions with receptor activity-modifying proteins. They also have therapeutic relevance for many conditions, such as osteoporosis, diabetes, obesity, lymphatic insufficiency, migraine and cardiovascular disease. In light of recent advances in understanding ligand docking and receptor activation in both the family as a whole and in CLR and CTR specifically, this review reflects how applicable general family B GPCR themes are to these two idiosyncratic receptors. We review the main functional domains of the receptors; the N-terminal extracellular domain, the juxtamembrane domain and ligand interface, the transmembrane domain and the intracellular C-terminal domain. Structural and functional findings from the CLR and CTR along with other family B GPCRs are critically appraised to gain insight into how these domains may function. The ability for CTR and CLR to interact with receptor activity-modifying proteins adds another level of sophistication to these receptor systems but means careful consideration is needed when trying to apply generic GPCR principles. This review encapsulates current thinking in the realm of family B GPCR research by highlighting both conflicting and recurring themes and how such findings relate to two unusual but important receptors, CTR and CLR.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

References
-
- Al-Sabah S, Donnelly D. The positive charge at Lys-288 of the glucagon-like peptide-1 (GLP-1) receptor is important for binding the N-terminus of peptide agonists. FEBS Lett. 2003;553:342–346. - PubMed
-
- Assil-Kishawi I, Abou-Samra AB. Sauvagine cross-links to the second extracellular loop of the corticotropin-releasing factor type 1 receptor. J Biol Chem. 2002;277:32558–32561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

