A titrate-to-goal study of switching patients uncontrolled on antihypertensive monotherapy to fixed-dose combinations of amlodipine and olmesartan medoxomil ± hydrochlorothiazide
- PMID: 21649839
- PMCID: PMC8108858
- DOI: 10.1111/j.1751-7176.2011.00437.x
A titrate-to-goal study of switching patients uncontrolled on antihypertensive monotherapy to fixed-dose combinations of amlodipine and olmesartan medoxomil ± hydrochlorothiazide
Abstract
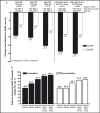
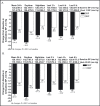
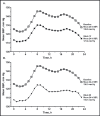
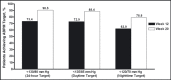
In the prospective, open-label, titrate-to-goal Blood Pressure Control in All Subgroups With Hypertension (BP-CRUSH) study, 999 patients with hypertension uncontrolled on monotherapy (mean age, 55.6 ± 11.4 years; baseline blood pressure [BP], 153.7 ± 9.2/91.9 ± 8.6 mm Hg) were switched to fixed-dose amlodipine/olmesartan medoxomil (AML/OM) 5/20 mg. Patients were uptitrated every 4 weeks to AML/OM 5/40 mg and 10/40 mg to achieve BP < 120/70 mm Hg. Patients were subsequently uptitrated every 4 weeks to AML/OM+hydrochlorothiazide (HCTZ) 10/40+12.5 mg and 10/40+25 mg to achieve BP <125/75 mm Hg. The primary end point, the cumulative percentage of patients achieving seated systolic BP < 140 mm Hg (< 130 mm Hg for patients with diabetes) by week 12, was 75.8%. The mean (± standard error) BP changes from baseline during the titration periods ranged from -14.2±0.4 mm Hg/-7.7 ± 0.3 mm Hg for AML/OM 5/20 mg to -25.1 ± 0.7 mm Hg/-13.7 ± 0.4 mm Hg for AML/OM+HCTZ 10/40+25 mg. By week 20, the cumulative BP threshold of <140/90 mm Hg was achieved by 90.3% of patients. An ambulatory BP monitoring substudy (n=243) showed that 24-hour efficacy was maintained. Treatment-emergent adverse events (TEAEs), mostly mild to moderate in severity, occurred in 529 patients (53.0%). Drug-related TEAEs occurred in 255 patients (25.5%). This well-tolerated, treat-to-goal algorithm enabled a large proportion of patients with uncontrolled hypertension on monotherapy to safely achieve BP control on single-pill AML/OM combination therapy or triple therapy with the addition of HCTZ. .
© 2011 Wiley Periodicals, Inc.
Figures

References
-
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. - PubMed
-
- Mori H, Ukai H, Yamamoto H, et al. Current status of antihypertensive prescription and associated blood pressure control in Japan. Hypertens Res. 2006;29(3):143–151. - PubMed
-
- Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289(16):2073–2082. - PubMed
-
- Qvarnström M, Wettermark B, Ljungman C, et al. Antihypertensive treatment and control in a large primary care population of 21 167 patients. J Hum Hypertens. 2010;Aug 19. [Epub ahead of print] - PubMed
-
- Spranger CB, Ries AJ, Berge CA, et al. Identifying gaps between guidelines and clinical practice in the evaluation and treatment of patients with hypertension. Am J Med. 2004;117(1):14–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

