CGG repeat in the FMR1 gene: size matters
- PMID: 21651511
- PMCID: PMC3151325
- DOI: 10.1111/j.1399-0004.2011.01723.x
CGG repeat in the FMR1 gene: size matters
Abstract
The FMR1 gene contains a CGG repeat present in the 5'-untranslated region which can be unstable upon transmission to the next generation. The repeat is up to 55 CGGs long in the normal population. In patients with fragile X syndrome (FXS), a repeat length exceeding 200 CGGs (full mutation: FM) generally leads to methylation of the repeat and the promoter region, which is accompanied by silencing of the FMR1 gene. The absence of FMR1 protein, FMRP, seen in FM is the cause of the mental retardation in patients with FXS. The premutation (PM) is defined as 55-200 CGGs. Female PM carriers are at risk of developing primary ovarian insufficiency. Elderly PM carriers might develop a progressive neurodegenerative disorder called fragile X-associated tremor/ataxia syndrome (FXTAS). Although arising from the mutations in the same gene, distinct mechanisms lead to FXS (absence of FMRP), FXTAS (toxic RNA gain-of-function) and FXPOI. The pathogenic mechanisms thought to underlie these disorders are discussed. This review gives insight on the implications of all possible repeat length categories seen in fragile X families.
2011 John Wiley & Sons A/S.
Conflict of interest statement
Conflict of interest: no
Figures

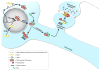

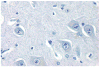

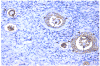
References
-
- Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. - PubMed
-
- Hagerman RJ. Physical and behavioral phenotype. In: Hagerman RJ, Silverman AC, editors. Fragile X syndrome: diagnosis, treatment and research. Baltimore and London: The John Hopkins University Press; 1996. pp. 3–87.
-
- Merenstein SA, Sobesky WE, Taylor AK, et al. Molecular-clinical correlations in males with an expanded FMR1 mutation. Am J Med Genet. 1996;64:388–394. - PubMed
-
- Yu S, Pritchard M, Kremer E, et al. Fragile X genotype characterized by an unstable region of DNA. Science. 1991;252:1179–1181. - PubMed
-
- Oberlé I, Rousseau F, Heitz D, et al. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

