Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities
- PMID: 21651512
- PMCID: PMC3187864
- DOI: 10.1111/j.1399-0004.2011.01724.x
Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities
Abstract
Rab GTPases are well-recognized targets in human disease, although are underexplored therapeutically. Elucidation of how mutant or dysregulated Rab GTPases and accessory proteins contribute to organ specific and systemic disease remains an area of intensive study and an essential foundation for effective drug targeting. Mutation of Rab GTPases or associated regulatory proteins causes numerous human genetic diseases. Cancer, neurodegeneration and diabetes represent examples of acquired human diseases resulting from the up- or downregulation or aberrant function of Rab GTPases. The broad range of physiologic processes and organ systems affected by altered Rab GTPase activity is based on pivotal roles in responding to cell signaling and metabolic demand through the coordinated regulation of membrane trafficking. The Rab-regulated processes of cargo sorting, cytoskeletal translocation of vesicles and appropriate fusion with the target membranes control cell metabolism, viability, growth and differentiation. In this review, we focus on Rab GTPase roles in endocytosis to illustrate normal function and the consequences of dysregulation resulting in human disease. Selected examples are designed to illustrate how defects in Rab GTPase cascades alter endocytic trafficking that underlie neurologic, lipid storage, and metabolic bone disorders as well as cancer. Perspectives on potential therapeutic modulation of GTPase activity through small molecule interventions are provided.
© 2011 John Wiley & Sons A/S.
Figures
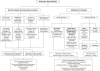




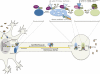
References
-
- Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. - PubMed
-
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

