High-level transgene expression by homologous recombination-mediated gene transfer
- PMID: 21652640
- PMCID: PMC3159483
- DOI: 10.1093/nar/gkr436
High-level transgene expression by homologous recombination-mediated gene transfer
Abstract
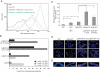
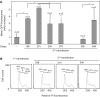
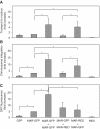
Gene transfer and expression in eukaryotes is often limited by a number of stably maintained gene copies and by epigenetic silencing effects. Silencing may be limited by the use of epigenetic regulatory sequences such as matrix attachment regions (MAR). Here, we show that successive transfections of MAR-containing vectors allow a synergistic increase of transgene expression. This finding is partly explained by an increased entry into the cell nuclei and genomic integration of the DNA, an effect that requires both the MAR element and iterative transfections. Fluorescence in situ hybridization analysis often showed single integration events, indicating that DNAs introduced in successive transfections could recombine. High expression was also linked to the cell division cycle, so that nuclear transport of the DNA occurs when homologous recombination is most active. Use of cells deficient in either non-homologous end-joining or homologous recombination suggested that efficient integration and expression may require homologous recombination-based genomic integration of MAR-containing plasmids and the lack of epigenetic silencing events associated with tandem gene copies. We conclude that MAR elements may promote homologous recombination, and that cells and vectors can be engineered to take advantage of this property to mediate highly efficient gene transfer and expression.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

