Evolutionary crossroads in developmental biology: sea urchins
- PMID: 21652646
- PMCID: PMC3109595
- DOI: 10.1242/dev.048967
Evolutionary crossroads in developmental biology: sea urchins
Abstract
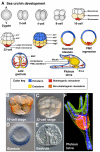
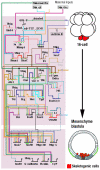
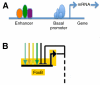
Embryos of the echinoderms, especially those of sea urchins and sea stars, have been studied as model organisms for over 100 years. The simplicity of their early development, and the ease of experimentally perturbing this development, provides an excellent platform for mechanistic studies of cell specification and morphogenesis. As a result, echinoderms have contributed significantly to our understanding of many developmental mechanisms, including those that govern the structure and design of gene regulatory networks, those that direct cell lineage specification, and those that regulate the dynamic morphogenetic events that shape the early embryo.
Figures



References
-
- Angerer L. M., Oleksyn D. W., Logan C. Y., McClay D. R., Dale L., Angerer R. C. (2000). A BMP pathway regulates cell fate allocation along the sea urchin animal-vegetal embryonic axis. Development 127, 1105-1114 - PubMed
-
- Angerer L. M., Oleksyn D. W., Levine A. M., Li X., Klein W. H., Angerer R. C. (2001). Sea urchin goosecoid function links fate specification along the animal-vegetal and oral-aboral embryonic axes. Development 128, 4393-4404 - PubMed
-
- Arnone M. I., Dmochowski I. J., Gache C. (2004). Using reporter genes to study cis-regulatory elements. Methods Cell Biol. 74, 621-652 - PubMed
-
- Boveri T. (1902). Uber mehrpolige mitosen als mittel zur analyse des zellkerns. Verh. d. phys.-med. Ges. Wursburg N. F. 35, 67-90
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

