Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction
- PMID: 21652678
- PMCID: PMC3156049
- DOI: 10.1182/blood-2010-11-321125
Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction
Abstract
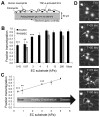
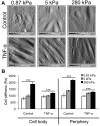
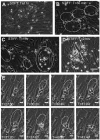
A vast amount of work has been dedicated to the effects of shear flow and cytokines on leukocyte transmigration. However, no studies have explored the effects of substrate stiffness on transmigration. Here, we investigated important aspects of endothelial cell contraction-mediated neutrophil transmigration using an in vitro model of the vascular endothelium. We modeled blood vessels of varying mechanical properties using fibronectin-coated polyacrylamide gels of varying physiologic stiffness, plated with human umbilical vein endothelial cell (HUVEC) monolayers, which were activated with tumor necrosis factor-α. Interestingly, neutrophil transmigration increased with increasing substrate stiffness below the endothelium. HUVEC intercellular adhesion molecule-1 expression, stiffness, cytoskeletal arrangement, morphology, and cell-substrate adhesion could not account for the dependence of transmigration on HUVEC substrate stiffness. We also explored the role of cell contraction and observed that large holes formed in endothelium on stiff substrates several minutes after neutrophil transmigration reached a maximum. Further, suppression of contraction through inhibition of myosin light chain kinase normalized the effects of substrate stiffness by reducing transmigration and eliminating hole formation in HUVECs on stiff substrates. These results provide strong evidence that neutrophil transmigration is regulated by myosin light chain kinase-mediated endothelial cell contraction and that this event depends on subendothelial cell matrix stiffness.
Figures




References
-
- Stroka KM, Aranda-Espinoza H. A biophysical view of the interplay between mechanical forces and signaling pathways during transendothelial cell migration. FEBS J. 2010;277(5):1145–1158. - PubMed
-
- Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33(5):1111–1117. - PubMed
-
- Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39(1):10–15. - PubMed
-
- Kothapalli D, Liu S, Byfield FJ, et al. Apolipoprotein E controls matrix protein synthesis and arterial stiffness.. Poster presented at the American Society of Cell Biology Annual Meeting; December 14, 2010; Philadelphia, PA.
-
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

