Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation
- PMID: 21652683
- PMCID: PMC3152492
- DOI: 10.1182/blood-2011-02-338665
Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation
Abstract
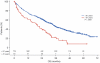
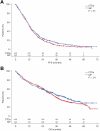
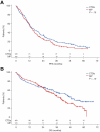
As part of the randomized MRC Myeloma IX trial, we compared an attenuated regimen of cyclophosphamide, thalidomide, and dexamethasone (CTDa; n = 426) with melphalan and prednisolone (MP; n = 423) in patients with newly diagnosed multiple myeloma ineligible for autologous stem-cell transplantation. The primary endpoints were overall response rate, progression-free survival, and overall survival (OS). The overall response rate was significantly higher with CTDa than MP (63.8% vs 32.6%; P < .0001), primarily because of increases in the rate of complete responses (13.1% vs 2.4%) and very good partial responses (16.9% vs 1.7%). Progression-free survival and OS were similar between groups. In this population, OS correlated with the depth of response (P < .0001) and favorable interphase fluorescence in situ hybridization profile (P < .001). CTDa was associated with higher rates of thromboembolic events, constipation, infection, and neuropathy than MP. In elderly patients with newly diagnosed multiple myeloma (median age, 73 years), CTDa produced higher response rates than MP but was not associated with improved survival outcomes. We highlight the importance of cytogenetic profiling at diagnosis and effective management of adverse events. This trial was registered at International Standard Randomized Controlled Trials Number as #68454111.
Figures



References
-
- Kumar S, Giralt S, Stadtmauer EA, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114(9):1729–1735. - PubMed
-
- Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2003;4(6):379–398. - PubMed
-
- Mileshkin L, Prince HM. The adverse prognostic impact of advanced age in multiple myeloma. Leuk Lymphoma. 2005;46(7):951–966. - PubMed
-
- Myeloma Trialists' Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. J Clin Oncol. 1998;16(12):3832–3842. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

