Stem cells and cell therapies in lung biology and lung diseases
- PMID: 21653527
- PMCID: PMC3132784
- DOI: 10.1513/pats.201012-071DW
Stem cells and cell therapies in lung biology and lung diseases
Abstract
The University of Vermont College of Medicine and the Vermont Lung Center, with support of the National Heart, Lung, and Blood Institute (NHLBI), the Alpha-1 Foundation, the American Thoracic Society, the Emory Center for Respiratory Health,the Lymphangioleiomyomatosis (LAM) Treatment Alliance,and the Pulmonary Fibrosis Foundation, convened a workshop,‘‘Stem Cells and Cell Therapies in Lung Biology and Lung Diseases,’’ held July 26-29, 2009 at the University of Vermont,to review the current understanding of the role of stem and progenitor cells in lung repair after injury and to review the current status of cell therapy approaches for lung diseases. These are rapidly expanding areas of study that provide further insight into and challenge traditional views of the mechanisms of lung repair after injury and pathogenesis of several lung diseases. The goals of the conference were to summarize the current state of the field, discuss and debate current controversies, and identify future research directions and opportunities for both basic and translational research in cell-based therapies for lung diseases.
Figures

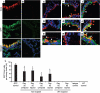


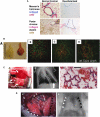



References
-
- Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc 2006;3:193–207. - PubMed
-
- Brown JK, Hogan BLM, Randell SH, Stripp B., Weiss DJ. Human embryonic stem cell research: An Official ATS Research Policy Statement. Am J Respir Crit Care Med 2006;173:1–3.
-
- Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 2007;87:858–870. - PubMed
-
- Flotte TR, Ng P, Dylla DE, McCray PB Jr, Wang G, Kolls JK, Hu J. Viral vector-mediated and cell-based therapies for treatment of cystic fibrosis. Mol Ther 2007;15:229–241. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
