Modules in the brain stem and spinal cord underlying motor behaviors
- PMID: 21653716
- PMCID: PMC3174810
- DOI: 10.1152/jn.00842.2010
Modules in the brain stem and spinal cord underlying motor behaviors
Abstract
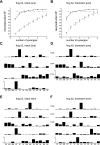
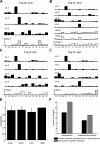
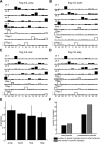
Previous studies using intact and spinalized animals have suggested that coordinated movements can be generated by appropriate combinations of muscle synergies controlled by the central nervous system (CNS). However, which CNS regions are responsible for expressing muscle synergies remains an open question. We address whether the brain stem and spinal cord are involved in expressing muscle synergies used for executing a range of natural movements. We analyzed the electromyographic (EMG) data recorded from frog leg muscles before and after transection at different levels of the neuraxis-rostral midbrain (brain stem preparations), rostral medulla (medullary preparations), and the spinal-medullary junction (spinal preparations). Brain stem frogs could jump, swim, kick, and step, while medullary frogs could perform only a partial repertoire of movements. In spinal frogs, cutaneous reflexes could be elicited. Systematic EMG analysis found two different synergy types: 1) synergies shared between pre- and posttransection states and 2) synergies specific to individual states. Almost all synergies found in natural movements persisted after transection at rostral midbrain or medulla but not at the spinal-medullary junction for swim and step. Some pretransection- and posttransection-specific synergies for a certain behavior appeared as shared synergies for other motor behaviors of the same animal. These results suggest that the medulla and spinal cord are sufficient for the expression of most muscle synergies in frog behaviors. Overall, this study provides further evidence supporting the idea that motor behaviors may be constructed by muscle synergies organized within the brain stem and spinal cord and activated by descending commands from supraspinal areas.
Figures
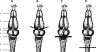

Similar articles
-
Muscle synergies encoded within the spinal cord: evidence from focal intraspinal NMDA iontophoresis in the frog.J Neurophysiol. 2001 Feb;85(2):605-19. doi: 10.1152/jn.2001.85.2.605. J Neurophysiol. 2001. PMID: 11160497
-
Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors.J Neurosci. 2005 Jul 6;25(27):6419-34. doi: 10.1523/JNEUROSCI.4904-04.2005. J Neurosci. 2005. PMID: 16000633 Free PMC article.
-
Modular premotor drives and unit bursts as primitives for frog motor behaviors.J Neurosci. 2004 Jun 2;24(22):5269-82. doi: 10.1523/JNEUROSCI.5626-03.2004. J Neurosci. 2004. PMID: 15175397 Free PMC article.
-
Approaches to revealing the neural basis of muscle synergies: a review and a critique.J Neurophysiol. 2021 May 1;125(5):1580-1597. doi: 10.1152/jn.00625.2019. Epub 2021 Mar 17. J Neurophysiol. 2021. PMID: 33729869 Review.
-
Isolated in vitro brainstem-spinal cord preparations remain important tools in respiratory neurobiology.Respir Physiol Neurobiol. 2012 Jan 15;180(1):1-7. doi: 10.1016/j.resp.2011.10.002. Epub 2011 Oct 12. Respir Physiol Neurobiol. 2012. PMID: 22015642 Free PMC article. Review.
Cited by
-
Evidence for altered upper extremity muscle synergies in chronic stroke survivors with mild and moderate impairment.Front Hum Neurosci. 2015 Feb 11;9:6. doi: 10.3389/fnhum.2015.00006. eCollection 2015. Front Hum Neurosci. 2015. PMID: 25717296 Free PMC article.
-
The Effects of Selective Muscle Weakness on Muscle Coordination in the Human Arm.Appl Bionics Biomech. 2018 Sep 19;2018:5637568. doi: 10.1155/2018/5637568. eCollection 2018. Appl Bionics Biomech. 2018. PMID: 30402139 Free PMC article.
-
Brainstem nucleus MdV mediates skilled forelimb motor tasks.Nature. 2014 Apr 17;508(7496):351-6. doi: 10.1038/nature13023. Epub 2014 Feb 2. Nature. 2014. PMID: 24487621
-
Pre-treatment EMG can be used to model post-treatment muscle coordination during walking in children with cerebral palsy.PLoS One. 2020 Feb 12;15(2):e0228851. doi: 10.1371/journal.pone.0228851. eCollection 2020. PLoS One. 2020. PMID: 32050002 Free PMC article.
-
A model-based approach to predict muscle synergies using optimization: application to feedback control.Front Comput Neurosci. 2015 Oct 6;9:121. doi: 10.3389/fncom.2015.00121. eCollection 2015. Front Comput Neurosci. 2015. PMID: 26500530 Free PMC article.
References
-
- Barale F, Corvaja N, Pompeiano O. Vestibular influences on postural activity in frog. Arch Ital Biol 109: 27–36, 1971 - PubMed
-
- Bizzi E, Mussa-Ivaldi FA, Giszter SF. Computations underlying the execution of movement: a biological perspective. Science 253: 287–291, 1991 - PubMed
-
- Cajigas-González I. Linear Control Model of the Spinal Processing of Descending Neural Signals (Master's thesis) Cambridge, MA: Massachusetts Inst. of Technology, 2003
-
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol 95: 3426–3437, 2006 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

