An infrequent molecular ruler controls flagellar hook length in Salmonella enterica
- PMID: 21654632
- PMCID: PMC3160246
- DOI: 10.1038/emboj.2011.185
An infrequent molecular ruler controls flagellar hook length in Salmonella enterica
Abstract
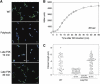
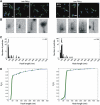
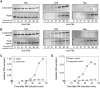
The bacterial flagellum consists of a long external filament connected to a membrane-embedded basal body at the cell surface by a short curved structure called the hook. In Salmonella enterica, the hook extends 55 nm from the cell surface. FliK, a secreted molecular ruler, controls hook length. Upon hook completion, FliK induces a secretion-specificity switch to filament-type substrate secretion. Here, we demonstrate that an infrequent ruler mechanism determines hook length. FliK is intermittently secreted during hook polymerization. The probability of the specificity switch is an increasing function of hook length. By uncoupling hook polymerization from FliK expression, we illustrate that FliK secretion immediately triggers the specificity switch in hooks greater than the physiological length. The experimental data display excellent agreement with a mathematical model of the infrequent ruler hypothesis. Merodiploid bacteria expressing simultaneously short and long ruler variants displayed hook-length control by the short ruler, further supporting the infrequent ruler model. Finally, the velocity of FliK secretion determines the probability of a productive FliK interaction with the secretion apparatus to change secretion substrate specificity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




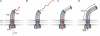
References
-
- Agrain C, Sorg I, Paroz C, Cornelis GR (2005) Secretion of YscP from Yersinia enterocolitica is essential to control the length of the injectisome needle but not to change the type III secretion substrate specificity. Mol Microbiol 57: 1415–1427 - PubMed
-
- Berg HC, Anderson RA (1973) Bacteria swim by rotating their flagellar filaments. Nature 245: 380–382 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

