The membrane bound LRR lipoprotein Slr, and the cell wall-anchored M1 protein from Streptococcus pyogenes both interact with type I collagen
- PMID: 21655249
- PMCID: PMC3105044
- DOI: 10.1371/journal.pone.0020345
The membrane bound LRR lipoprotein Slr, and the cell wall-anchored M1 protein from Streptococcus pyogenes both interact with type I collagen
Abstract
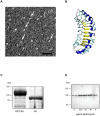
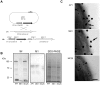
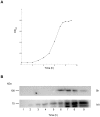
Streptococcus pyogenes is an important human pathogen and surface structures allow it to adhere to, colonize and invade the human host. Proteins containing leucine rich repeats (LRR) have been identified in mammals, viruses, archaea and several bacterial species. The LRRs are often involved in protein-protein interaction, are typically 20-30 amino acids long and the defining feature of the LRR motif is an 11-residue sequence LxxLxLxxNxL (x being any amino acid). The streptococcal leucine rich (Slr) protein is a hypothetical lipoprotein that has been shown to be involved in virulence, but at present no ligands for Slr have been identified. We could establish that Slr is a membrane attached horseshoe shaped lipoprotein by homology modeling, signal peptidase II inhibition, electron microscopy (of bacteria and purified protein) and immunoblotting. Based on our previous knowledge of LRR proteins we hypothesized that Slr could mediate binding to collagen. We could show by surface plasmon resonance that recombinant Slr and purified M1 protein bind with high affinity to collagen I. Isogenic slr mutant strain (MB1) and emm1 mutant strain (MC25) had reduced binding to collagen type I as shown by slot blot and surface plasmon resonance. Electron microscopy using gold labeled Slr showed multiple binding sites to collagen I, both to the monomeric and the fibrillar structure, and most binding occurred in the overlap region of the collagen I fibril. In conclusion, we show that Slr is an abundant membrane bound lipoprotein that is co-expressed on the surface with M1, and that both these proteins are involved in recruiting collagen type I to the bacterial surface. This underlines the importance of S. pyogenes interaction with extracellular matrix molecules, especially since both Slr and M1 have been shown to be virulence factors.
Conflict of interest statement
Figures




References
-
- Kreikemeyer B, Nakata M, Oehmcke S, Gschwendtner C, Normann J, et al. Streptococcus pyogenes collagen type I-binding Cpa surface protein. Expression profile, binding characteristics, biological functions, and potential clinical impact. J Biol Chem. 2005;280:33228–33239. - PubMed
-
- Jin MS, Lee JO. Structures of TLR-ligand complexes. Curr Opin Immunol. 2008;20:414–419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

