Immunolocalization of influenza A virus and markers of inflammation in the human Parkinson's disease brain
- PMID: 21655265
- PMCID: PMC3105060
- DOI: 10.1371/journal.pone.0020495
Immunolocalization of influenza A virus and markers of inflammation in the human Parkinson's disease brain
Abstract
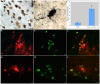
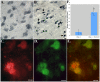
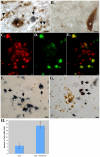
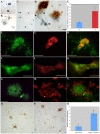
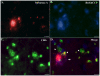
Although much is known regarding the molecular mechanisms leading to neuronal cell loss in Parkinson's disease (PD), the initiating event has not been identified. Prevailing theories including a chemical insult or infectious agent have been postulated as possible triggers, leading to neuroinflammation. We present immunohistochemical data indicating the presence of influenza A virus within the substantia nigra pars compacta (SNpc) from postmortem PD brain sections. Influenza A virus labeling was identified within neuromelanin granules as well as on tissue macrophages in the SNpc. Further supporting a role for neuroinflammation in PD was the identification of T-lymphocytes that colocalized with an antibody to caspase-cleaved Beclin-1 within the SNpc. The presence of influenza A virus together with macrophages and T-lymphocytes may contribute to the neuroinflammation associated with this disease.
Conflict of interest statement
Figures

Similar articles
-
Parkinson's disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta.Mov Disord. 2017 Mar;32(3):441-449. doi: 10.1002/mds.26883. Epub 2016 Dec 22. Mov Disord. 2017. PMID: 28004859
-
Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease.Neuroreport. 2006 Jul 31;17(11):1215-8. doi: 10.1097/01.wnr.0000227984.84927.a7. Neuroreport. 2006. PMID: 16837857
-
Combined enrichment of neuromelanin granules and synaptosomes from human substantia nigra pars compacta tissue for proteomic analysis.J Proteomics. 2013 Dec 6;94:202-206. doi: 10.1016/j.jprot.2013.07.015. Epub 2013 Aug 3. J Proteomics. 2013. PMID: 23917253 Free PMC article. Clinical Trial.
-
The enigma of neuromelanin in Parkinson's disease substantia nigra.J Neural Transm Suppl. 1994;43:113-22. J Neural Transm Suppl. 1994. PMID: 7884393 Review.
-
Iron as the concert master in the pathogenic orchestra playing in sporadic Parkinson's disease.J Neural Transm (Vienna). 2021 Oct;128(10):1577-1598. doi: 10.1007/s00702-021-02414-z. Epub 2021 Oct 12. J Neural Transm (Vienna). 2021. PMID: 34636961 Free PMC article. Review.
Cited by
-
Infections and Changes in Commensal Bacteria and the Pathogenesis of Parkinson's Disease.J Parkinsons Dis. 2022;12(s1):S45-S51. doi: 10.3233/JPD-223271. J Parkinsons Dis. 2022. PMID: 35723116 Free PMC article. Review.
-
Parkinsonism as a Third Wave of the COVID-19 Pandemic?J Parkinsons Dis. 2020;10(4):1343-1353. doi: 10.3233/JPD-202211. J Parkinsons Dis. 2020. PMID: 32986683 Free PMC article.
-
Infectious agents and neurodegeneration.Mol Neurobiol. 2012 Dec;46(3):614-38. doi: 10.1007/s12035-012-8320-7. Epub 2012 Aug 17. Mol Neurobiol. 2012. PMID: 22899188 Free PMC article. Review.
-
Infectious Etiologies of Parkinsonism: Pathomechanisms and Clinical Implications.Front Neurol. 2019 Jun 19;10:652. doi: 10.3389/fneur.2019.00652. eCollection 2019. Front Neurol. 2019. PMID: 31275235 Free PMC article. Review.
-
Post-COVID-19 Parkinsonism and Parkinson's Disease Pathogenesis: The Exosomal Cargo Hypothesis.Int J Mol Sci. 2022 Aug 28;23(17):9739. doi: 10.3390/ijms23179739. Int J Mol Sci. 2022. PMID: 36077138 Free PMC article. Review.
References
-
- Reichmann H. Clinical criteria for the diagnosis of Parkinson's disease. Neurodegener Dis. 2010;7:284–290. - PubMed
-
- Dickson DW. Alpha-synuclein and the Lewy body disorders. Curr Opin Neurol. 2001;14:423–432. - PubMed
-
- Ravenholt RT, Foege WH. 1918 influenza, encephalitis lethargica, parkinsonism. Lancet. 1982;2:860–864. - PubMed
-
- Kumar A, Calne SM, Schulzer M, Mak E, Wszolek Z, et al. Clustering of Parkinson disease: shared cause or coincidence? Arch Neurol. 2004;61:1057–1060. - PubMed
-
- Goldsmith JR, Herishanu YO, Podgaietski M, Kordysh E. Dynamics of parkinsonism-Parkinson's disease in residents of adjacent kibbutzim in Israel's Negev. Environ Res. 1997;73:156–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

