A template-dependent dislocation mechanism potentiates K65R reverse transcriptase mutation development in subtype C variants of HIV-1
- PMID: 21655292
- PMCID: PMC3105016
- DOI: 10.1371/journal.pone.0020208
A template-dependent dislocation mechanism potentiates K65R reverse transcriptase mutation development in subtype C variants of HIV-1
Abstract
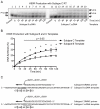
Numerous studies have suggested that the K65R reverse transcriptase (RT) mutation develops more readily in subtype C than subtype B HIV-1. We recently showed that this discrepancy lies partly in the subtype C template coding sequence that predisposes RT to pause at the site of K65R mutagenesis. However, the mechanism underlying this observation and the elevated rates of K65R development remained unknown. Here, we report that DNA synthesis performed with subtype C templates consistently produced more K65R-containing transcripts than subtype B templates, regardless of the subtype-origin of the RT enzymes employed. These findings confirm that the mechanism involved is template-specific and RT-independent. In addition, a pattern of DNA synthesis characteristic of site-specific primer/template slippage and dislocation was only observed with the subtype C sequence. Analysis of RNA secondary structure suggested that the latter was unlikely to impact on K65R development between subtypes and that Streisinger strand slippage during DNA synthesis at the homopolymeric nucleotide stretch of the subtype C K65 region might occur, resulting in misalignment of the primer and template. Consequently, slippage would lead to a deletion of the middle adenine of codon K65 and the production of a -1 frameshift mutation, which upon dislocation and realignment of the primer and template, would lead to development of the K65R mutation. These findings provide additional mechanistic evidence for the facilitated development of the K65R mutation in subtype C HIV-1.
Conflict of interest statement
Figures








References
-
- Osmanov S, Pattou C, Walker N, Schwardlander B, Esparza J. Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J Acquir Immune Defic Syndr. 2002;29:184–190. - PubMed
-
- Heeney JL, Dalgleish AG, Weiss RA. Origins of HIV and the evolution of resistance to AIDS. Science. 2006;313:462–466. - PubMed
-
- Plantier JC, Leoz M, Dickerson JE, De Oliveira F, Cordonnier F, et al. A new human immunodeficiency virus derived from gorillas. Nat Med. 2009;15:871–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

