N-terminal acetylation inhibits protein targeting to the endoplasmic reticulum
- PMID: 21655302
- PMCID: PMC3104963
- DOI: 10.1371/journal.pbio.1001073
N-terminal acetylation inhibits protein targeting to the endoplasmic reticulum
Abstract
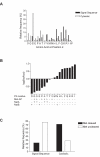
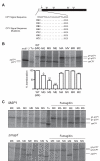
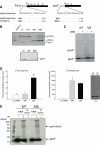
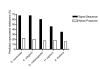
Amino-terminal acetylation is probably the most common protein modification in eukaryotes with as many as 50%-80% of proteins reportedly altered in this way. Here we report a systematic analysis of the predicted N-terminal processing of cytosolic proteins versus those destined to be sorted to the secretory pathway. While cytosolic proteins were profoundly biased in favour of processing, we found an equal and opposite bias against such modification for secretory proteins. Mutations in secretory signal sequences that led to their acetylation resulted in mis-sorting to the cytosol in a manner that was dependent upon the N-terminal processing machinery. Hence N-terminal acetylation represents an early determining step in the cellular sorting of nascent polypeptides that appears to be conserved across a wide range of species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Martinez A, Traverso J. A, Valot B, Ferro M, Espagne C, et al. Extent of N-terminal modifications in cytosolic proteins from eukaryotes. Proteomics. 2008;8:2809–2831. - PubMed
-
- Arfin S. M, Bradshaw R. A. Cotranslational processing and protein turnover in eukaryotic cells. Biochemistry. 1988;27:7979–7984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

