Liver damage and systemic inflammatory responses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia
- PMID: 21656186
- PMCID: PMC3224647
- DOI: 10.1007/s11302-011-9239-6
Liver damage and systemic inflammatory responses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia
Abstract
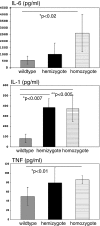
Liver ischemia reperfusion injury is associated with both local damage to the hepatic vasculature and systemic inflammatory responses. CD39 is the dominant vascular endothelial cell ectonucleotidase and rapidly hydrolyses both adenosine triphosphate (ATP) and adenosine diphosphate to adenosine monophosphate. These biochemical properties, in tandem with 5'-nucleotidases, generate adenosine and potentially illicit inflammatory vascular responses and thrombosis. We have evaluated the role of CD39 in total hepatic ischemia reperfusion injury (IRI). Wildtype mice, Cd39-hemizygous mice (+/-) and matched Cd39-null mice (-/-); (n = 25 per group) underwent 45 min of total warm ischemia with full inflow occlusion necessitating partial hepatectomy. Soluble nucleoside triphosphate diphosphohydrolase (NTPDases) or adenosine/amrinone were administered to wildtype (n = 6) and Cd39-null mice (n = 6) in order to study protective effects in vivo. Parameters of liver injury, systemic inflammation, hepatic ATP determinations by P(31)-NMR and parameters of lung injury were obtained. All wildtype mice survived up to 7 days with minimal biochemical disturbances and minor evidence for injury. In contrast, 64% of Cd39+/- and 84% of Cd39-null mice required euthanasia or died within 4 h post-reperfusion with liver damage and systemic inflammation associated with hypercytokinemia. Hepatic ATP depletion was pronounced in Cd39-null mice posthepatic IRI. Soluble NTPDase or adenosine administration protected Cd39-deficient mice from acute reperfusion injury. We conclude that CD39 is protective in hepatic IRI preventing local injury and systemic inflammation in an adenosine dependent manner. Our data indicate that vascular CD39 expression has an essential protective role in hepatic IRI.
Figures






References
-
- Thiagarajan RR, Winn RK, Harlan JM. The role of leukocyte and endothelial adhesion molecules in ischemia-reperfusion injury. Thromb Haemost. 1997;78:310–314. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

