Prostate-derived Ets transcription factor (PDEF) is a potential prognostic marker in patients with prostate cancer
- PMID: 21656828
- PMCID: PMC3112264
- DOI: 10.1002/pros.21333
Prostate-derived Ets transcription factor (PDEF) is a potential prognostic marker in patients with prostate cancer
Abstract
Background: Reduced expression of prostate-derived Ets transcription factor (PDEF) leads to morphologic change as well as increased migration and invasiveness of prostate cancer cells. However, the clinical relevance of PDEF expression and its relationship to anti-apoptotic protein survivin is yet to be determined.
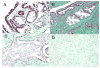
Methods: Tissue microarrays of 73 prostate carcinomas and their adjacent benign prostate tissue, as well as 50 benign prostates were evaluated for PDEF expression by immunohistochemistry. Results were confirmed in available tumor tissues using Western blot and RT-PCR. Expression of survivin in prostate carcinoma and benign tissues were determined using Western blot. Results and correlation with clinical data were statistically analyzed.
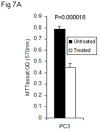
Results: Patients' specimens with low Gleason scores (GS < 5) expressed higher levels of PDEF protein and lower levels of survivin protein when compared with moderate-to-high GS tumors (GS > 6). Patients with PDEF-positive tumor survived significantly longer (P < 0.0001) than patients with PDEF-negative tumor, and the 8-year survival rate was 94% and 40%, respectively. PDEF expression was detected at the highest levels in benign tissues and was down-regulated or lost in 30 recently diagnosed prostate carcinomas. Re-expression of PDEF in prostate cancer cells inhibited survivin expression. Treatment of prostate cancer cells with methylseleninic acid resulted in restoration of PDEF expression, down-regulation of survivin, and inhibition of tumor cell growth when compared with untreated controls (P < 0.05).
Conclusions: These studies demonstrated an inverse correlation between PDEF and survivin expression, and that up-regulation of PDEF was associated with a favorable prognosis in patients with clinically localized prostate cancer.
Copyright © 2011 Wiley-Liss, Inc.
Figures














References
-
- Oettgen P, Finger E, Sun Z, Akbarali Y, Thamrongsak U, Boltax J, Grall F, Dube A, Weiss A, Brown L, Quinn G, Kas K, Endress G, Kunsch C, Libermann TA. PDEF, a novel prostate epithelium-specific transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J Biol Chem. 2000;275:1216–1225. - PubMed
-
- Kas K, Finger E, Grall F, Gu X, Akbarali Y, Boltax J, Weiss A, Oettgen P, Kapeller R, Libermann TA. ESE-3, a novel member of an epithelium-specific ets transcription factor subfamily, demonstrates different target gene specificity from ESE-1. J Biol Chem. 2000;275:2986–98. - PubMed
-
- Gu X, Zerbini LF, Otu HH, Bhasin M, Yang Q, Joseph MG, Grall F, Onatunde T, Correa RG, Libermann TA. Reduced PDEF expression increases invasion and expression of mesenchymal genes in prostate cancer cells. Cancer Res. 2007;67:4219–4226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

