The SirT3 divining rod points to oxidative stress
- PMID: 21658599
- PMCID: PMC3526939
- DOI: 10.1016/j.molcel.2011.05.008
The SirT3 divining rod points to oxidative stress
Abstract
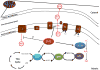
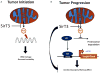
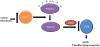
Sirtuins are NAD(+) dependent deacetylases that counter aging and diseases of aging. Sirtuin research has focused on SirT1, which deacetylates transcription factors and cofactors in the nucleus. More recent findings highlight SirT3 as a mitochondrial sirtuin that regulates metabolism and oxidative stress. This review focuses on new data linking SirT3 to management of reactive oxygen species from mitochondria, which may have profound implications for aging and late-onset diseases.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

