Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats
- PMID: 21658845
- PMCID: PMC3181388
- DOI: 10.1016/j.psyneuen.2011.04.015
Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats
Abstract
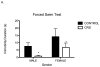
Nearly 12% of US children are exposed to intense adverse experiences. Research has demonstrated that these experiences can negatively impact adult health, often resulting in psychopathology. Less attention, however, is given to the impact of childhood adverse experiences on childhood health and wellbeing. Using a rodent model of chronic juvenile stress (restraint 6 h daily from postnatal day 20 to 41), we report that chronic stress has significant immediate morbidities in both males and females during this developmental window. Specifically, we demonstrate that chronic juvenile stress produces depressive-like behavior and significant neuronal remodeling of brain regions likely involved in these behavioral alterations: the hippocampus, prefrontal cortex and amygdala. Chronically stressed males and females exhibit anhedonia, increased locomotion when exposed to novelty, and altered coping strategies when exposed to acute stress. Coincident with these behavioral changes, we report simplification of dendrites in the hippocampus and prefrontal cortex and concurrent hypertrophy of dendrites in the amygdala. Taken together, these results demonstrate that chronically stressed juveniles exhibit aberrant behavioral responses to acute challenges that occur in conjunction with stress-induced remodeling of brain regions intimately involved in regulating emotionality and stress reactivity. Further, the absence of sex differences in our reported stress responses, likely speaks to the decreased sensitivity of immature HPA regulating brain regions to sex hormones.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
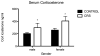

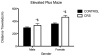




References
-
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. xii. Washington, D.C: American Psychiatric Association; 2000. p. 370.
-
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29(5):1043–53. - PubMed
-
- Apter D. Development of the hypothalamic-pituitary-ovarian axis. Ann N Y Acad Sci. 1997;816:9–21. - PubMed
-
- Barha CK, Brummelte S, Lieblich SE, Galea LA. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus 2010 - PubMed
-
- Bhatia SK, Bhatia SC. Childhood and adolescent depression. Am Fam Physician. 2007;75(1):73–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

