Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated β-cell death
- PMID: 21659501
- PMCID: PMC3142078
- DOI: 10.2337/db10-1643
Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated β-cell death
Abstract
Objective: CD4 T-cells secreting interleukin (IL)-17 are implicated in several human autoimmune diseases, but their role in type 1 diabetes has not been defined. To address the relevance of such cells, we examined IL-17 secretion in response to β-cell autoantigens, IL-17A gene expression in islets, and the potential functional consequences of IL-17 release for β-cells.
Research design and methods: Peripheral blood CD4 T-cell responses to β-cell autoantigens (proinsulin, insulinoma-associated protein, and GAD65 peptides) were measured by IL-17 enzyme-linked immunospot assay in patients with new-onset type 1 diabetes (n = 50). mRNA expression of IL-17A and IFNG pathway genes was studied by qRT-PCR using islets obtained from subjects who died 5 days and 10 years after diagnosis of disease, respectively, and from matched control subjects. IL-17 effects on the function of human islets, rat β-cells, and the rat insulinoma cell line INS-1E were examined.
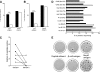
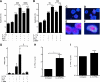
Results: A total of 27 patients (54%) showed IL-17 reactivity to one or more β-cell peptides versus 3 of 30 (10%) control subjects (P = 0.0001). In a single case examined close to diagnosis, islet expression of IL17A, RORC, and IL22 was detected. It is noteworthy that we show that IL-17 mediates significant and reproducible enhancement of IL-1β/interferon (IFN)-γ-induced and tumor necrosis factor (TNF)-α/IFN-γ-induced apoptosis in human islets, rat β-cells, and INS-1E cells, in association with significant upregulation of β-cell IL17RA expression via activation of the transcription factors STAT1 and nuclear factor (NF)-κB.
Conclusions: Circulating IL-17(+) β-cell-specific autoreactive CD4 T-cells are a feature of type 1 diabetes diagnosis. We disclose a novel pathway to β-cell death involving IL-17 and STAT1 and NF-κB, rendering this cytokine a novel disease biomarker and potential therapeutic target.
Figures

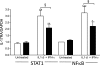

References
-
- Frisullo G, Nociti V, Iorio R, et al. IL17 and IFNgamma production by peripheral blood mononuclear cells from clinically isolated syndrome to secondary progressive multiple sclerosis. Cytokine 2008;44:22–25 - PubMed
-
- Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 2002;8:500–508 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

