Defective photoreceptor phagocytosis in a mouse model of enhanced S-cone syndrome causes progressive retinal degeneration
- PMID: 21659555
- PMCID: PMC3157681
- DOI: 10.1096/fj.11-186767
Defective photoreceptor phagocytosis in a mouse model of enhanced S-cone syndrome causes progressive retinal degeneration
Abstract
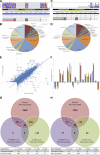
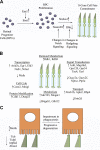
Enhanced S-cone syndrome (ESCS), featuring an excess number of S cones, manifests as a progressive retinal degeneration that leads to blindness. Here, through optical imaging, we identified an abnormal interface between photoreceptors and the retinal pigment epithelium (RPE) in 9 patients with ESCS. The neural retina leucine zipper transcription factor-knockout (Nrl(-/-)) mouse model demonstrates many phenotypic features of human ESCS, including unstable S-cone-positive photoreceptors. Using massively parallel RNA sequencing, we identified 6203 differentially expressed transcripts between wild-type (Wt) and Nrl(-/-) mouse retinas, with 6 highly significant differentially expressed genes of the Pax, Notch, and Wnt canonical pathways. Changes were also obvious in expression of 30 genes involved in the visual cycle and 3 key genes in photoreceptor phagocytosis. Novel high-resolution (100 nm) imaging and reconstruction of Nrl(-/-) retinas revealed an abnormal packing of photoreceptors that contributed to buildup of photoreceptor deposits. Furthermore, lack of phagosomes in the RPE layer of Nrl(-/-) retina revealed impairment in phagocytosis. Cultured RPE cells from Wt and Nrl(-/-) mice illustrated that the phagocytotic defect was attributable to the aberrant interface between ESCS photoreceptors and the RPE. Overcoming the retinal phagocytosis defect could arrest the progressive degenerative component of this disease.
Figures





References
-
- Livesey F. J., Cepko C. L. (2001) Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2, 109–118 - PubMed
-
- Anderson D. H., Fisher S. K., Steinberg R. H. (1978) Mammalian cones: disc shedding, phagocytosis, and renewal. Invest. Ophthalmol. Vis. Sci. 17, 117–133 - PubMed
-
- Immel J. H., Fisher S. K. (1985) Cone photoreceptor shedding in the tree shrew (tupaia-belangerii). Cell Tissue Res. 239, 667–675 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

