Shifting responsibly: the importance of striatal modularity to reinforcement learning in uncertain environments
- PMID: 21660099
- PMCID: PMC3105240
- DOI: 10.3389/fnhum.2011.00047
Shifting responsibly: the importance of striatal modularity to reinforcement learning in uncertain environments
Abstract
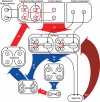
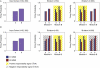
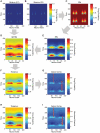
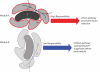
We propose here that the modular organization of the striatum reflects a context-sensitive modular learning architecture in which clustered striosome-matrisome domains participate in modular reinforcement learning (RL). Based on anatomical and physiological evidence, it has been suggested that the modular organization of the striatum could represent a learning architecture. There is not, however, a coherent view of how such a learning architecture could relate to the organization of striatal outputs into the direct and indirect pathways of the basal ganglia, nor a clear formulation of how such a modular architecture relates to the RL functions attributed to the striatum. Here, we hypothesize that striosome-matrisome modules not only learn to bias behavior toward specific actions, as in standard RL, but also learn to assess their own relevance to the environmental context and modulate their own learning and activity on this basis. We further hypothesize that the contextual relevance or "responsibility" of modules is determined by errors in predictions of environmental features and that such responsibility is assigned by striosomes and conveyed to matrisomes via local circuit interneurons. To examine these hypotheses and to identify the general requirements for realizing this architecture in the nervous system, we developed a simple modular RL model. We then constructed a network model of basal ganglia circuitry that includes these modules and the direct and indirect pathways. Based on simple assumptions, this model suggests that while the direct pathway may promote actions based on striatal action values, the indirect pathway may act as a gating network that facilitates or suppresses behavioral modules on the basis of striatal responsibility signals. Our modeling functionally unites the modular compartmental organization of the striatum with the direct-indirect pathway divisions of the basal ganglia, a step that we suggest will have important clinical implications.
Keywords: acetylcholine; basal ganglia; direct and indirect pathways; mixture of experts; modular reinforcement learning; responsibility signal; striatum; striosome and matrix compartments.
Figures
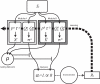
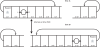
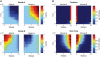
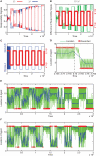
References
-
- Alexander G. E., DeLong M. R., Strick P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381 - PubMed
-
- Amemori K., Graybiel A. M. (2009). “Stimulation of the macaque rostral anterior cingulate cortex alters decision in approach-avoidance conflict,” in Program No. 194.1, 2009 Neuroscience Meeting Planner (Chicago, IL: Society for Neuroscience; ).
-
- Amemori K., Graybiel A. M. (2010). “Localized microstimulation of macaque pregenual anterior cingulate cortex increases rejection of cued outcomes in approach-avoidance decision-making,” in Program No. 306.4, 2010 Neuroscience Meeting Planner (San Diego, CA: Society for Neuroscience; ).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

