Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection
- PMID: 21660934
- PMCID: PMC3517125
- DOI: 10.1002/eji.201041363
Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection
Abstract
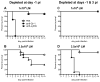
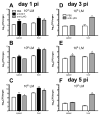
Previous studies have suggested that neutrophils are required for resistance during infection with multiple pathogenic microorganisms. However, the depleting antibody used in those studies binds to both Ly6G and Ly6C (anti-Gr-1; clone RB6-8C5). This antibody has been shown to deplete not only neutrophils but also monocytes and a subset of CD8(+) T cells. Recently, an antibody against Ly6G, which specifically depletes neutrophils, was characterized. In the present study, neutrophils are depleted using the antibody against Ly6G during infection with the intracellular bacterium Listeria monocytogenes (LM). Our data show that neutrophil-depleted mice are much less susceptible to infection than mice depleted with anti-Gr-1. Although neutrophils are required for clearance of LM, their importance is more pronounced in the liver and during a high-dose bacterial challenge. Furthermore, we demonstrate that the protection mediated by neutrophils is due to the production of TNF-α, but not IFN-γ. Additionally, neutrophils are not required for the recruitment of monocytes or the generation of adaptive T-cell responses during LM infection. This study highlights the importance of neutrophils during LM infection, and indicate that depletion of neutrophils is less detrimental to the host than depletion of all Gr-1-expressing cell populations.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures

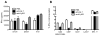


Comment in
-
Revisiting the protective and pathogenic roles of neutrophils: Ly-6G is key!Eur J Immunol. 2011 Sep;41(9):2535-8. doi: 10.1002/eji.201141979. Eur J Immunol. 2011. PMID: 21952813
References
-
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. - PubMed
-
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. - PubMed
-
- Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. - PubMed
-
- Rogers HW, Callery MP, Deck B, Unanue ER. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

