Monodisperse magnetic nanoparticles for theranostic applications
- PMID: 21661754
- PMCID: PMC3184307
- DOI: 10.1021/ar200090c
Monodisperse magnetic nanoparticles for theranostic applications
Abstract
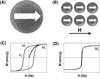
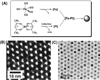
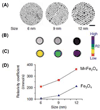
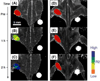
Effective medical care requires the concurrent monitoring of medical treatment. The combination of imaging and therapeutics allows a large degree of control over the treatment efficacy and is now commonly referred to as "theranostics". Magnetic nanoparticles (NPs) provide a unique nanoplatform for theranostic applications because of their biocompatibility, their responses to the external magnetic field, and their sizes which are comparable to that of functional biomolecules. Recent studies of magnetic NPs for both imaging and therapeutic applications have led to greater control over size, surface functionalization, magnetic properties, and specific binding capabilities of the NPs. The combination of the deep tissue penetration of the magnetic field and the ability of magnetic NPs to enhance magnetic resonance imaging sensitivity and magnetic heating efficiency makes magnetic NPs promising candidates for successful future theranostics. In this Account, we review recent advances in the synthesis of magnetic NPs for biomedical applications such as magnetic resonance imaging (MRI) and magnetic fluid hyperthermia (MFH). Our focus is on iron oxide (Fe(3)O(4)) NPs, gold-iron oxide (Au-Fe(3)O(4)) NPs, metallic iron (Fe) NPs, and Fe-based alloy NPs, such as iron-cobalt (FeCo) and iron-platinum (FePt) NPs. Because of the ease of fabrication and their approved clinical usage, Fe(3)O(4) NPs with controlled sizes and surface chemistry have been studied extensively for MRI and MFH applications. Porous hollow Fe(3)O(4) NPs are expected to have similar magnetic, chemical, and biological properties as the solid Fe(3)O(4) NPs, and their structures offer the additional opportunity to store and release drugs at a target. The Au-Fe(3)O(4) NPs combine both magnetically active Fe(3)O(4) and optically active Au within one nanostructure and are a promising NP platform for multimodal imaging and therapeutics. Metallic Fe and FeCo NPs offer the opportunity for probes with even higher magnetizations. However, metallic NPs are normally very reactive and are subject to fast oxidation in biological solutions. Once they are coated with a layer of polycrystalline Fe(3)O(4) or a graphitic shell, these metallic NPs are more stable and provide better contrast for MRI and more effective heating for MFH. FePt NPs are chemically more stable than Fe and FeCo NPs and have shown great potential as contrast agents for both MRI and X-ray computed tomography (CT) and as robust probes for controlled heating in MFH.
Figures



References
-
- Warner S. Diagnostics plus therapy = theranostics. Scientist. 2004;18(16):38–39.
-
- Murray CB, Kagan CR, Bawendi MG. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu Rev Mater Sci. 2000;30:545–610.
-
- Kim J, Piao Y, Hyeon T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem Soc Rev. 2009;38(2):372–390. - PubMed
-
- Xie J, Huang J, Li X, Sun S, Chen X. Iron oxide nanoparticle platform for biomedical applications. Curr Med Chem. 2009;16(10):1278–1294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

