Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults
- PMID: 21663486
- PMCID: PMC3171707
- DOI: 10.3109/00365521.2011.584895
Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults
Abstract
Objective: To assess the impact of Bifidobacterium lactis HN019 supplementation on whole gut transit time (WGTT) and frequency of functional gastrointestinal (GI) symptoms in adults.
Material and methods: We randomized 100 subjects (mean age: 44 years; 64% female) with functional GI symptoms to consume a proprietary probiotic strain, B. lactis HN019 (Fonterra Research Centre, Palmerston North, New Zealand), at daily doses of 17.2 billion colony forming units (CFU) (high dose; n = 33), 1.8 billion CFU (low dose; n = 33), or placebo (n = 34) for 14 days. The primary endpoint of WGTT was assessed by X-ray on days 0 and 14 and was preceded by consumption of radiopaque markers once a day for 6 days. The secondary endpoint of functional GI symptom frequency was recorded with a subject-reported numeric (1-100) scale before and after supplementation.
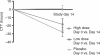
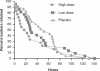
Results: Decreases in mean WGTT over the 14-day study period were statistically significant in the high dose group (49 ± 30 to 21 ± 32 h, p < 0.001) and the low dose group (60 ± 33 to 41 ± 39 h, p = 0.01), but not in the placebo group (43 ± 31 to 44 ± 33 h). Time to excretion of all ingested markers was significantly shorter in the treatment groups versus placebo. Of the nine functional GI symptoms investigated, eight significantly decreased in frequency in the high dose group and seven decreased with low dose, while two decreased in the placebo group. No adverse events were reported in any group.
Conclusions: Daily B. lactis HN019 supplementation is well tolerated, decreases WGTT in a dose-dependent manner, and reduces the frequency of functional GI symptoms in adults.
Figures



References
-
- Drossman DA. Introduction. The Rome Foundation and Rome III. Neurogastroenterol Motil. 2007;19:783–6. - PubMed
-
- Locke GR, 3rd, Zinsmeister AR, Fett SL, Melton LJ, 3rd, Talley NJ. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil. 2005;17:29–34. - PubMed
-
- Haider SL, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799–807. - PubMed
-
- Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–80. - PubMed
-
- Talley NJ, Weaver AL, Zinsmeister AR, Melton LJ., 3rd Onset and disappearance of gastrointestinal symptoms and functional gastrointestinal disorders. Am J Epidemiol. 1992;136:165–77. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
