A conserved juxtacrine signal regulates synaptic partner recognition in Caenorhabditis elegans
- PMID: 21663630
- PMCID: PMC3130637
- DOI: 10.1186/1749-8104-6-28
A conserved juxtacrine signal regulates synaptic partner recognition in Caenorhabditis elegans
Abstract
Background: An essential stage of neural development involves the assembly of neural circuits via formation of inter-neuronal connections. Early steps in neural circuit formation, including cell migration, axon guidance, and the localization of synaptic components, are well described. However, upon reaching their target region, most neurites still contact many potential partners. In order to assemble functional circuits, it is critical that within this group of cells, neurons identify and form connections only with their appropriate partners, a process we call synaptic partner recognition (SPR). To understand how SPR is mediated, we previously developed a genetically encoded fluorescent trans-synaptic marker called NLG-1 GRASP, which labels synaptic contacts between individual neurons of interest in dense cellular environments in the genetic model organism Caenorhabditis elegans.
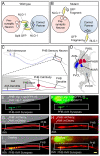
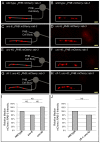
Results: Here, we describe the first use of NLG-1 GRASP technology, to identify SPR genes that function in this critical process. The NLG-1 GRASP system allows us to assess synaptogenesis between PHB sensory neurons and AVA interneurons instantly in live animals, making genetic analysis feasible. Additionally, we employ a behavioral assay to specifically test PHB sensory circuit function. Utilizing this approach, we reveal a new role for the secreted UNC-6/Netrin ligand and its transmembrane receptor UNC-40/Deleted in colorectal cancer (DCC) in SPR. Synapses between PHB and AVA are severely reduced in unc-6 and unc-40 animals despite normal axon guidance and subcellular localization of synaptic components. Additionally, behavioral defects indicate a complete disruption of PHB circuit function in unc-40 mutants. Our data indicate that UNC-40 and UNC-6 function in PHB and AVA, respectively, to specify SPR. Strikingly, overexpression of UNC-6 in postsynaptic neurons is sufficient to promote increased PHB-AVA synaptogenesis and to potentiate the behavioral response beyond wild-type levels. Furthermore, an artificially membrane-tethered UNC-6 expressed in the postsynaptic neurons promotes SPR, consistent with a short-range signal between adjacent synaptic partners.
Conclusions: These results indicate that the conserved UNC-6/Netrin-UNC-40/DCC ligand-receptor pair has a previously unknown function, acting in a juxtacrine manner to specify recognition of individual postsynaptic neurons. Furthermore, they illustrate the potential of this new approach, combining NLG-1 GRASP and behavioral analysis, in gene discovery and characterization.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

