In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics
- PMID: 21664664
- PMCID: PMC3124562
- DOI: 10.1016/j.cell.2011.05.024
In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics
Abstract
Neurogenesis and gliogenesis continue in discrete regions of the adult mammalian brain. A fundamental question remains whether cell genesis occurs from distinct lineage-restricted progenitors or from self-renewing and multipotent neural stem cells in the adult brain. Here, we developed a genetic marking strategy for lineage tracing of individual, quiescent, and nestin-expressing radial glia-like (RGL) precursors in the adult mouse dentate gyrus. Clonal analysis identified multiple modes of RGL activation, including asymmetric and symmetric self-renewal. Long-term lineage tracing in vivo revealed a significant percentage of clones that contained RGL(s), neurons, and astrocytes, indicating capacity of individual RGLs for both self-renewal and multilineage differentiation. Furthermore, conditional Pten deletion in RGLs initially promotes their activation and symmetric self-renewal but ultimately leads to terminal astrocytic differentiation and RGL depletion in the adult hippocampus. Our study identifies RGLs as self-renewing and multipotent neural stem cells and provides novel insights into in vivo properties of adult neural stem cells.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
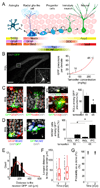
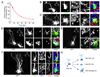
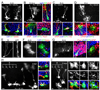

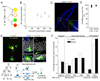


Comment in
-
The pessimist's and optimist's views of adult neurogenesis.Cell. 2011 Jun 24;145(7):1009-11. doi: 10.1016/j.cell.2011.06.011. Cell. 2011. PMID: 21703445
References
-
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. - PubMed
-
- Bronner-Fraser M, Fraser SE. Cell lineage analysis of the avian neural crest. Development. 1991 Suppl 2:17–22. - PubMed
-
- Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

