Protein folding and quality control in the endoplasmic reticulum: Recent lessons from yeast and mammalian cell systems
- PMID: 21664808
- PMCID: PMC3154734
- DOI: 10.1016/j.ceb.2011.05.004
Protein folding and quality control in the endoplasmic reticulum: Recent lessons from yeast and mammalian cell systems
Abstract
The evolution of eukaryotes was accompanied by an increased need for intracellular communication and cellular specialization. Thus, a more complex collection of secreted and membrane proteins had to be synthesized, modified, and folded. The endoplasmic reticulum (ER) thereby became equipped with devoted enzymes and associated factors that both catalyze the production of secreted proteins and remove damaged proteins. A means to modify ER function to accommodate and destroy misfolded proteins also evolved. Not surprisingly, a growing number of human diseases are linked to various facets of ER function. Each of these topics will be discussed in this article, with an emphasis on recent reports in the literature that employed diverse models.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

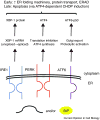
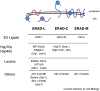
 . Select yeast factors (in parentheses) required for the degradation of each class are listed, and mammalian homologs are indicated in cases in which these have also been shown to function during ERAD, although not necessarily in a class-specific manner. Note: Many mammalian E3s have been identified as contributing to the ERAD of select factors (e.g., CHIP, HRD1, RMA1, gp78, TEB4, and Parkin), but for simplicity these are not shown. In addition, there is evidence that Hsp70 and its constitutively expressed isoform, Hsc70, may play unique roles during protein folding and ERAD, respectively [153].
. Select yeast factors (in parentheses) required for the degradation of each class are listed, and mammalian homologs are indicated in cases in which these have also been shown to function during ERAD, although not necessarily in a class-specific manner. Note: Many mammalian E3s have been identified as contributing to the ERAD of select factors (e.g., CHIP, HRD1, RMA1, gp78, TEB4, and Parkin), but for simplicity these are not shown. In addition, there is evidence that Hsp70 and its constitutively expressed isoform, Hsc70, may play unique roles during protein folding and ERAD, respectively [153].References
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
-
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. - PubMed
-
- Crowley KS, Liao S, Worrell VE, Reinhart GD, Johnson AE. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 1994;78:461–471. - PubMed
-
- Woolhead CA, McCormick PJ, Johnson AE. Nascent membrane and secretory proteins differ in FRET-detected folding far inside the ribosome and in their exposure to ribosomal proteins. Cell. 2004;116:725–736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

