Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture
- PMID: 21666010
- PMCID: PMC3147449
- DOI: 10.1128/AEM.00637-11
Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture
Abstract
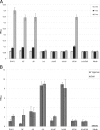
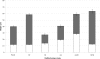
Pseudomonas aeruginosa is an opportunistic human pathogen and has been established as a model organism to study bacterial biofilm formation. At least three exopolysaccharides (alginate, Psl, and Pel) contribute to the formation of biofilms in this organism. Here mutants deficient in the production of one or more of these polysaccharides were generated to investigate how these polymers interactively contribute to biofilm formation. Confocal laser scanning microscopy of biofilms formed in flow chambers showed that mutants deficient in alginate biosynthesis developed biofilms with a decreased proportion of viable cells than alginate-producing strains, indicating a role of alginate in viability of cells in biofilms. Alginate-deficient mutants showed enhanced extracellular DNA (eDNA)-containing surface structures impacting the biofilm architecture. PAO1 ΔpslA Δalg8 overproduced Pel, and eDNA showing meshwork-like structures presumably based on an interaction between both polymers were observed. The formation of characteristic mushroom-like structures required both Psl and alginate, whereas Pel appeared to play a role in biofilm cell density and/or the compactness of the biofilm. Mutants producing only alginate, i.e., mutants deficient in both Psl and Pel production, lost their ability to form biofilms. A lack of Psl enhanced the production of Pel, and the absence of Pel enhanced the production of alginate. The function of Psl in attachment was independent of alginate and Pel. A 30% decrease in Psl promoter activity in the alginate-overproducing MucA-negative mutant PDO300 suggested inverse regulation of both biosynthesis operons. Overall, this study demonstrated that the various exopolysaccharides and eDNA interactively contribute to the biofilm architecture of P. aeruginosa.
Figures



References
-
- Allesen-Holm M., et al. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59:1114–1128 - PubMed
-
- Choi K. H., Kumar A., Schweizer H. P. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64:391–397 - PubMed
-
- Friedman L., Kolter R. 2004. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51:675–690 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

