Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization
- PMID: 21666057
- PMCID: PMC3131424
- DOI: 10.4049/jimmunol.1002701
Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization
Abstract
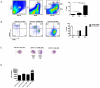
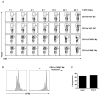
Although several subsets of intestinal APCs have been described, there has been no systematic evaluation of their phenotypes, functions, and regional localization to date. In this article, we used 10-color flow cytometry to define the major APC subsets in the small and large intestine lamina propria. Lamina propria APCs could be subdivided into CD11c(+)CD11b(-), CD11c(+)CD11b(+), and CD11c(dull)CD11b(+) subsets. CD11c(+)CD11b(-) cells were largely CD103(+)F4/80(-) dendritic cells (DCs), whereas the CD11c(+)CD11b(+) subset comprised CD11c(+)CD11b(+)CD103(+)F4/80(-) DCs and CD11c(+)CD11b(+)CD103(-)F4/80(+) macrophage-like cells. The majority of CD11c(dull)CD11b(+) cells were CD103(-)F4/80(+) macrophages. Although macrophages were more efficient at inducing Foxp3(+) regulatory T (T(reg)) cells than DCs, at higher T cell/APC ratios, all of the DC subsets efficiently induced Foxp3(+) T(reg) cells. In contrast, only CD11c(+)CD11b(+)CD103(+) DCs efficiently induced Th17 cells. Consistent with this, the regional distribution of CD11c(+)CD11b(+)CD103(+) DCs correlated with that of Th17 cells, with duodenum > jejunum > ileum > colon. Conversely, CD11c(+)CD11b(-)CD103(+) DCs, macrophages, and Foxp3(+) T(reg) cells were most abundant in the colon and scarce in the duodenum. Importantly, however, the ability of DC and macrophage subsets to induce Foxp3(+) T(reg) cells versus Th17 cells was strikingly dependent on the source of the mouse strain. Thus, DCs from C57BL/6 mice from Charles River Laboratories (that have segmented filamentous bacteria, which induce robust levels of Th17 cells in situ) were more efficient at inducing Th17 cells and less efficient at inducing Foxp3(+) T(reg) cells than DCs from B6 mice from The Jackson Laboratory. Thus, the functional specializations of APC subsets in the intestine are dependent on the T cell/APC ratio, regional localization, and source of the mouse strain.
Figures
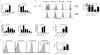





References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. - PubMed
-
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. - PubMed
Publication types
MeSH terms
Grants and funding
- R37 AI048638/AI/NIAID NIH HHS/United States
- T32 GM008169/GM/NIGMS NIH HHS/United States
- AI50019/AI/NIAID NIH HHS/United States
- R01 DK057665/DK/NIDDK NIH HHS/United States
- AI0564499/AI/NIAID NIH HHS/United States
- R37 DK057665/DK/NIDDK NIH HHS/United States
- R01 AI048638/AI/NIAID NIH HHS/United States
- DK057665/DK/NIDDK NIH HHS/United States
- N01 AI050019/AI/NIAID NIH HHS/United States
- AA01787001/AA/NIAAA NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
- R21 AI083554/AI/NIAID NIH HHS/United States
- AI083554/AI/NIAID NIH HHS/United States
- AI048638/AI/NIAID NIH HHS/United States
- AI05726601/AI/NIAID NIH HHS/United States
- AI057157/AI/NIAID NIH HHS/United States
- R56 AI048638/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

