Influence of a subtype of inhibitory interneuron on stimulus-specific responses in visual cortex
- PMID: 21666125
- PMCID: PMC3278313
- DOI: 10.1093/cercor/bhr057
Influence of a subtype of inhibitory interneuron on stimulus-specific responses in visual cortex
Abstract
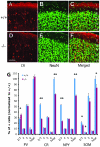
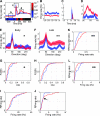
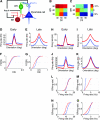
Inhibition modulates receptive field properties and integrative responses of neurons in cortical circuits. The contribution of specific interneuron classes to cortical circuits and emergent responses is unknown. Here, we examined neuronal responses in primary visual cortex (V1) of adult Dlx1(-/-) mice, which have a selective reduction in cortical dendrite-targeting interneurons (DTIs) that express calretinin, neuropeptide Y, and somatostatin. The V1 neurons examined in Dlx1(-/-) mice have reduced orientation selectivity and altered firing rates, with elevated late responses, suggesting that local inhibition at dendrites has a specific role in modulating neuronal computations. We did not detect overt changes in the physiological properties of thalamic relay neurons and features of thalamocortical projections, such as retinotopic maps and eye-specific inputs, in the mutant mice, suggesting that the defects are cortical in origin. These experimental results are well explained by a computational model that integrates broad tuning from dendrite-targeting and narrower tuning from soma-targeting interneuron subclasses. Our findings suggest a key role for DTIs in the fine-tuning of stimulus-specific cortical responses.
Figures



Similar articles
-
Chronic reduction in inhibition reduces receptive field size in mouse auditory cortex.Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13829-34. doi: 10.1073/pnas.1205909109. Epub 2012 Jul 2. Proc Natl Acad Sci U S A. 2012. PMID: 22753490 Free PMC article.
-
Inhibitory stabilization and visual coding in cortical circuits with multiple interneuron subtypes.J Neurophysiol. 2016 Mar;115(3):1399-409. doi: 10.1152/jn.00732.2015. Epub 2016 Jan 6. J Neurophysiol. 2016. PMID: 26740531 Free PMC article.
-
Cortical Control of Spatial Resolution by VIP+ Interneurons.J Neurosci. 2016 Nov 9;36(45):11498-11509. doi: 10.1523/JNEUROSCI.1920-16.2016. J Neurosci. 2016. PMID: 27911754 Free PMC article.
-
How inhibitory circuits in the thalamus serve vision.Annu Rev Neurosci. 2015 Jul 8;38:309-29. doi: 10.1146/annurev-neuro-071013-014229. Annu Rev Neurosci. 2015. PMID: 26154979 Review.
-
Understanding layer 4 of the cortical circuit: a model based on cat V1.Cereb Cortex. 2003 Jan;13(1):73-82. doi: 10.1093/cercor/13.1.73. Cereb Cortex. 2003. PMID: 12466218 Review.
Cited by
-
Age-related changes to layer 3 pyramidal cells in the rhesus monkey visual cortex.Cereb Cortex. 2015 Jun;25(6):1454-68. doi: 10.1093/cercor/bht336. Epub 2013 Dec 8. Cereb Cortex. 2015. PMID: 24323499 Free PMC article.
-
DLX genes and proteins in mammalian forebrain development.Development. 2024 Jun 1;151(11):dev202684. doi: 10.1242/dev.202684. Epub 2024 May 31. Development. 2024. PMID: 38819455 Free PMC article. Review.
-
Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants.J Comp Neurol. 2013 May 1;521(7):1561-84. doi: 10.1002/cne.23242. J Comp Neurol. 2013. PMID: 23042297 Free PMC article.
-
Long-term down-regulation of GABA decreases orientation selectivity without affecting direction selectivity in mouse primary visual cortex.Front Neural Circuits. 2013 Mar 25;7:28. doi: 10.3389/fncir.2013.00028. eCollection 2013. Front Neural Circuits. 2013. PMID: 23533083 Free PMC article.
-
Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders.Mol Psychiatry. 2019 Sep;24(9):1248-1257. doi: 10.1038/s41380-019-0426-0. Epub 2019 May 14. Mol Psychiatry. 2019. PMID: 31089192 Free PMC article. Review.
References
-
- Allison JD, Kabara JF, Snider RK, Casagrande VA, Bonds AB. GABAB-receptor-mediated inhibition reduces the orientation selectivity of the sustained response of striate cortical neurons in cats. Vis Neurosci. 1996;13(3):559–566. - PubMed
-
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84(2):909–926. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278(5337):474–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

