High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments
- PMID: 21666667
- PMCID: PMC3152576
- DOI: 10.1038/nmeth.1630
High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments
Abstract
To quantitatively understand chemosensory behaviors, it is desirable to present many animals with repeatable, well-defined chemical stimuli. To that end, we describe a microfluidic system to analyze Caenorhabditis elegans behavior in defined temporal and spatial stimulus patterns. A 2 cm × 2 cm structured arena allowed C. elegans to perform crawling locomotion in a controlled liquid environment. We characterized behavioral responses to attractive odors with three stimulus patterns: temporal pulses, spatial stripes and a linear concentration gradient, all delivered in the fluid phase to eliminate variability associated with air-fluid transitions. Different stimulus configurations preferentially revealed turning dynamics in a biased random walk, directed orientation into an odor stripe and speed regulation by odor. We identified both expected and unexpected responses in wild-type worms and sensory mutants by quantifying dozens of behavioral parameters. The devices are inexpensive, easy to fabricate, reusable and suitable for delivering any liquid-borne stimulus.
Figures
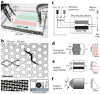





References
-
- Robinson DA. The use of control systems analysis in the neurophysiology of eye movements. Annu Rev Neurosci. 1981;4:463–503. - PubMed
-
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–27. - PubMed
-
- Chalasani SH, et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

