C/EBP-δ regulates VEGF-C autocrine signaling in lymphangiogenesis and metastasis of lung cancer through HIF-1α
- PMID: 21666710
- PMCID: PMC3175299
- DOI: 10.1038/onc.2011.187
C/EBP-δ regulates VEGF-C autocrine signaling in lymphangiogenesis and metastasis of lung cancer through HIF-1α
Abstract
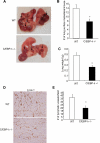
CCAAT/enhancer-binding protein-δ (C/EBP-δ), a transcription factor, is elevated in carcinoma compared with that in normal tissue. This study reports a novel function of C/EBP-δ in lymphangiogenesis and tumor metastasis. Genetic deletion of C/EBP-δ in mice resulted in a significant reduction of lymphangiogenesis and pulmonary metastases, with a dramatic reduction of vascular endothelial growth factor-C (VEGF-C) and its cognate receptor VEGF receptor-3 (VEGFR3) in lymphatic endothelial cells (LECs). By contrast, no difference of VEGF-C in tumor tissues and bone marrow was observed between null and wild-type mice. Consistently, forced expression of C/EBP-δ increased VEGF-C and VEGFR3 expression in cultured LECs. These findings suggest a specific and important role of C/EBP-δ in the regulation of VEGFR3 signaling in LECs. Furthermore, expression of C/EBP-δ in cultured LECs significantly increased cell motility, and knockdown of C/EBP-δ inhibited cell motility and lymphatic vascular network formation in vitro. Forced expression of VEGF-C, but not recombinant VEGF-C, rescued the knockdown of C/EBP-δ-induced cell apoptosis, indicative of autonomous VEGF-C autocrine signaling essential for LEC survival. Moreover, hypoxia induces C/EBP-δ expression and C/EBP-δ regulates HIF-1α expression. Blocking HIF-1α activity totally blocked CEBP-δ-induced VEGF-C and VEGFR3 expression in LECs. Together, these findings uncover a new function of CEBP-δ in lymphangiogenesis through regulation of VEGFR3 signaling in LECs.
Figures




References
-
- Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. - PubMed
-
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. - PubMed
-
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. - PubMed
-
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

