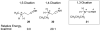Superelectrophilic chemistry of amino-nitriles and related substrates
- PMID: 21666850
- PMCID: PMC3110710
- DOI: 10.1016/j.tet.2011.04.038
Superelectrophilic chemistry of amino-nitriles and related substrates
Abstract
The superacid-promoted Houben-Hoesch reactions of amino-nitriles and related compounds have been studied. The nitriles form dicationic electrophiles and react with benzene in fair to good yields (12-95%). The intermediate iminium ions may also be reduced to the benzylic amines by NaBH(4) or H(2).
Figures
References
-
- Hoesch K. Ber. Dtsch. Chem. Ges. 1915;48:1122.
- Houben J. Ber. Dtsch. Chem. Ges. 1926;59:2878.
- Smith MB, March J. March’s Advanced Organic Chemistry. 6th Ed. Wiley; NY: 2007. pp. 732–733.
-
- Sato Y, Yato M, Ohwada T, Saito S, Shudo K. J. Am. Chem. Soc. 1995;117:3037.
- Yato M, Ohwada T, Shudo K. J. Am. Chem. Soc. 1991;113:691.
-
- Klumpp DA. In: Recent Developments in Carbocation and Onium Ion Chemistry. Laali K, editor. American Chemical Society; Washington, DC: 2007. pp. 144–159. ACS Symposium Series 395.
-
- Gracias V, Ji Z, Akritopoulou-Zanze I, Abad-Zapatero C, Huth JR, Song D, Hajduk PJ, Johnson EF, Glaser KB, Marcotte PA, Pease L, Soni NB, Stewart KD, Davidsen SK, Michaelides MR, Djuric SW. Bioorg. Med. Chem. Lett. 2008;18:2691. - PubMed
- Raja AS, Pandeya SN, Panda SS, Stables JP. Pharm. Chem. J. 2007;41:302.
-
- Chung F, Tisne C, Lecourt T, Dardel F, Micouin L. Angew. Chem. Int. Ed. 2007;46:4489. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources