Tetrabenazine inhibition of monoamine uptake and methamphetamine behavioral effects: locomotor activity, drug discrimination and self-administration
- PMID: 21669212
- PMCID: PMC3780357
- DOI: 10.1016/j.neuropharm.2011.05.033
Tetrabenazine inhibition of monoamine uptake and methamphetamine behavioral effects: locomotor activity, drug discrimination and self-administration
Abstract
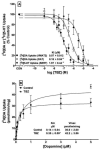
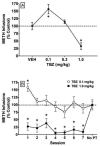
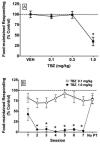
Tetrabenazine (TBZ), a benzoquinolizine derivative, binds with high affinity to the vesicular monoamine transporter-2 (VMAT2), inhibiting uptake of cytosolic monoamines. The current study aimed to provide preclinical evidence supporting the potential use of TBZ as a treatment for methamphetamine abuse. Effects of TBZ on function of the dopamine transporter (DAT) and serotonin transporter (SERT) in striatal and hippocampal synaptosomes, respectively, and on VMAT2 function in isolated striatal synaptic vesicles were determined. Effect of TBZ (acute, 0.1-3.0 mg/kg, s.c.; repeated, 1.0 mg/kg for 7 days) on locomotor activity in methamphetamine-sensitized rats was assessed. Ability of TBZ (0.1-3.0 mg/kg; s.c.) or vehicle to decrease the discriminative effect of methamphetamine also was determined. Ability of TBZ (acute, 0.1-1.0 mg/kg, s.c.; repeated, 0.1 or 1.0 mg/kg for 7 days) to specifically decrease methamphetamine self-administration was determined; for comparison, a separate group of rats was assessed for effects of TBZ on food-maintained responding. Results show that TBZ was 11-fold more potent inhibiting DAT than SERT, and 2.5-fold more potent inhibiting VMAT2 than DAT. Results from behavioral studies showed that the lowest dose of TBZ transiently increased methamphetamine self-administration, whereas higher TBZ doses decreased methamphetamine self-administration. Also, TBZ at high doses decreased methamphetamine locomotor sensitization and discriminative stimulus effects, as well as food-maintained responding. Thus, despite acting as a potent VMAT2 inhibitor, these preclinical results indicate that TBZ lacks behavioral specificity as an inhibitor of methamphetamine-induced reinforcement, diminishing its viability as a suitable treatment for methamphetamine abuse.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Bohnen NI, Minoshima S, Koeppe RA, Meyer P, Wenette K, Kilbourn MR, Kuhl DE, Frey KA, Albin RL. Decreased striatal monaminergic terminals in Huntington's Disease. Neurology. 2000;54:1753–1759. - PubMed
-
- Brown JM, Hanson GR, Fleckenstein AE. Methamphetamine rapidly decreases vesicular dopamine uptake. J Neurochem. 2000;74:2221–2223. - PubMed
-
- Brown JM, Hanson GR, Fleckenstein AE. Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J Pharmacol Exp Ther. 2001;296:762–767. - PubMed
-
- Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63:89–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

