Retinoic acid signaling sequentially controls visceral and heart laterality in zebrafish
- PMID: 21669875
- PMCID: PMC3151095
- DOI: 10.1074/jbc.M111.244327
Retinoic acid signaling sequentially controls visceral and heart laterality in zebrafish
Abstract
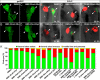
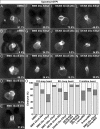
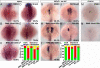
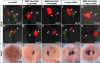
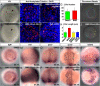
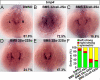
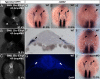
During zebrafish development, the left-right (LR) asymmetric signals are first established around the Kupffer vesicle (KV), a ciliated organ generating directional fluid flow. Then, LR asymmetry is conveyed and stabilized in the lateral plate mesoderm. Although numerous molecules and signaling pathways are involved in controlling LR asymmetry, mechanistic difference and concordance between different organs during LR patterning are poorly understood. Here we show that RA signaling regulates laterality decisions at two stages in zebrafish. Before the 2-somite stage (2So), inhibition of RA signaling leads to randomized visceral laterality through bilateral expression of nodal/spaw in the lateral plate mesoderm, which is mediated by increases in cilia length and defective directional fluid flow in KV. Fgf8 is required for the regulation of cilia length by RA signaling. Blockage of RA signaling before 2So also leads to mild defects of heart laterality, which become much more severe through perturbation of cardiac bmp4 asymmetry when RA signaling is blocked after 2So. At this stage, visceral laterality and the left-sided Nodal remain unaffected. These findings suggest that RA signaling controls visceral laterality through the left-sided Nodal signal before 2So, and regulates heart laterality through cardiac bmp4 mainly after 2So, first identifying sequential control and concordance of visceral and heart laterality.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

