The spatio-temporal characteristics of action potential initiation in layer 5 pyramidal neurons: a voltage imaging study
- PMID: 21669974
- PMCID: PMC3180577
- DOI: 10.1113/jphysiol.2011.209015
The spatio-temporal characteristics of action potential initiation in layer 5 pyramidal neurons: a voltage imaging study
Abstract
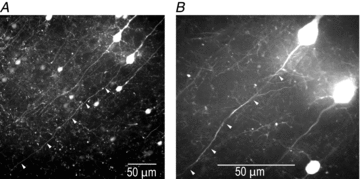
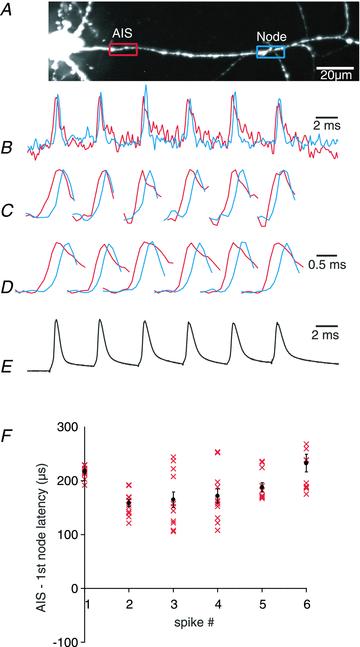
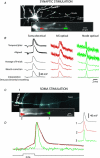
The spatial pattern of Na(+) channel clustering in the axon initial segment (AIS) plays a critical role in tuning neuronal computations, and changes in Na(+) channel distribution have been shown to mediate novel forms of neuronal plasticity in the axon. However, immunocytochemical data on channel distribution may not directly predict spatio-temporal characteristics of action potential initiation, and prior electrophysiological measures are either indirect (extracellular) or lack sufficient spatial resolution (intracellular) to directly characterize the spike trigger zone (TZ). We took advantage of a critical methodological improvement in the high sensitivity membrane potential imaging (V(m) imaging) technique to directly determine the location and length of the spike TZ as defined in functional terms. The results show that in mature axons of mouse cortical layer 5 pyramidal cells, action potentials initiate in a region ∼20 μm in length centred between 20 and 40 μm from the soma. From this region, the AP depolarizing wave invades initial nodes of Ranvier within a fraction of a millisecond and propagates in a saltatory fashion into axonal collaterals without failure at all physiologically relevant frequencies. We further demonstrate that, in contrast to the saltatory conduction in mature axons, AP propagation is non-saltatory (monotonic) in immature axons prior to myelination.
Figures
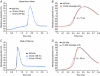





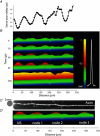

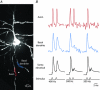
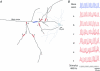
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

