BMP4 promotes prostate tumor growth in bone through osteogenesis
- PMID: 21670081
- PMCID: PMC3148283
- DOI: 10.1158/0008-5472.CAN-10-4374
BMP4 promotes prostate tumor growth in bone through osteogenesis
Abstract
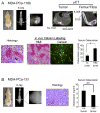
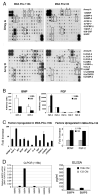
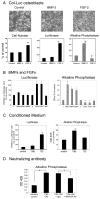
Induction of new bone formation is frequently seen in the bone lesions from prostate cancer. However, whether osteogenesis is necessary for prostate tumor growth in bone is unknown. Recently, 2 xenografts, MDA-PCa-118b and MDA-PCa-133, were generated from prostate cancer bone metastases. When implanted subcutaneously in severe combined immunodeficient (SCID) mice, MDA-PCa-118b induced strong ectopic bone formation while MDA-PCa-133 did not. To identify the factors that are involved in bone formation, we compared the expression of secreted factors (secretome) from MDA-PCa-118b and MDA-PCa-133 by cytokine array. We found that the osteogenic MDA-PCa-118b xenograft expressed higher levels of bone morphogenetic protein BMP4 and several cytokines including interleukin-8, growth-related protein (GRO), and CCL2. We showed that BMP4 secreted from MDA-PCa-118b contributed to about a third of the osteogenic differentiation seen in MDA-PCa-118b tumors. The conditioned media from MDA-PCa-118b induced a higher level of osteoblast differentiation, which was significantly reduced by treatment with BMP4 neutralizing antibody or the small molecule BMP receptor 1 inhibitor LDN-193189. BMP4 did not elicit an autocrine effect on MDA-PCa-118b, which expressed low to undetectable levels of BMP receptors. Treatment of SCID mice bearing MDA-PCa-118b tumors with LDN-193189 significantly reduced tumor growth. Thus, these studies support a role of BMP4-mediated osteogenesis in the progression of prostate cancer in bone.
Figures



References
-
- Jacobs SC. Spread of prostatic cancer to bone. Urology. 1983;21:337–44. - PubMed
-
- Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–16. - PubMed
-
- Saad F, Lipton A. Bone-marker levels in patients with prostate cancer: potential correlations with outcomes. Curr Opin Support Palliat Care. 2010;4:127–34. - PubMed
-
- Logothetis C, Lin S-H. Osteoblasts in prostate cancer metastasis to bone. Nature Reviews Cancer. 2005;5:21–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

